(協作聚測試沙盒)
| 工具箱 |
下列各项的文字格式及显示效果都在模板消息列表中,請点击展開
(以下带*号者,请加签名~~~~作结)
|
| |
協作目標:甲状腺素
- 原始:0位元組
- 沙盒:25,381位元組
来源搜索:「"甲狀腺素"」——Google:网页、新闻、学术、图书、图片;百度:网页、新闻、学术、图片;知网工具书;JSTOR;维基百科图书馆
| 聚會/Wiki協作聚/沙盒/甲狀腺素 | |
|---|---|
| |

| |
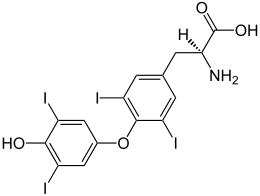
| IUPAC名 (2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]propanoic acid | |
| 识别 | |
| CAS号 | 7488-70-2 |
| PubChem | 853 |
| SMILES |
|
| MeSH | Thyroxine |
| 性质 | |
| 化学式 | C15H11I4NO4 |
| 摩尔质量 | 776.87 g·mol⁻¹ |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
甲状腺素是甲狀腺激素之一,由酪胺酸和碘所組合而成的兒茶酚胺類激素,分為三碘甲狀腺素(T3)及四碘甲狀腺素(T4),T3的效果大概比T4強3到4倍。
碘為構成甲状腺素的主要成分,當缺乏時會造成甲状腺合成减少,引起甲状腺組織肿胀,即「缺碘性甲狀腺腫」,俗称「大脖子病」。甲狀腺素有促进细胞代谢,增加氧消耗,刺激组织生长、成熟和分化的功能,並且有助於腸道中葡萄糖的吸收。血液循環中的甲状腺素大多為半生期較長的T4。T3和T4在血液中的比例大約為1比20,而T4在細胞中會脫碘酶(deiodinase)轉為活性較強的T3,並更進一步脫羧及脫碘形成3-碘類甲腺質(T1a)及類甲腺質 (T0a)。三種脫碘酶皆為含硒元素的酵素,因此硒元素的攝取在T3的製造上十分重要。
甲状腺功能亢进时,基础代谢增加造成內分泌旺盛,會有以下生理特徵:頭痛、神經緊張、心跳及呼吸加速、體重減輕、失眠、手抖、多汗、怕熱、疲倦、凸眼、消化不良、腹瀉等問題,需要減少對甲狀腺素主要物質碘的攝取量。
甲状腺功能低下時,會有以下生理特徵:體重上升怕冷、疲倦、嗜睡、體重增加、水腫、精神遲鈍等症狀。
东周时,中国已经有了关于甲状腺病的记载,在晋朝时,已经知道了用海藻可以治疗此病。
功能 编辑
甲狀腺素幾乎在身體上所有細胞作用,影響包括提高基礎代謝率、影響蛋白質合成、協同生長激素造成骨骼的生長、促進神經的成熟以及提高身體對兒茶酚胺類激素(如腎上腺素)的敏感性。
甲狀腺素在正常發育及細胞分化上扮演了重要的角色,它控制了蛋白質、脂肪以及碳水化合物的代謝,並且刺激維生素的代謝。數種生理上及病理上刺激影響了甲狀腺素的合成。
製造 编辑
中樞 编辑
- 甲狀腺球蛋白在粗糙內質網中合成,並經由胞吐作用進入到甲狀腺濾泡腔的膠質中。
-同時,鈉-碘幫浦經由主動運輸將碘離子(I-)由血液唧入細胞
-碘離子利用碘離子通道經由促進性擴散進入濾泡腔[5]
-碘離子(I-)在膠質中被甲狀腺過氧化酶(thyroid peroxidase)氧化為碘元素(I0)
-碘元素(I0)碘化了球蛋白的酪胺醯基(約120個).
-隨後,毗鄰的酪胺醯殘基兩兩結合
- 複合體經由胞吐作用進入到了濾泡腔
- 蛋白酶將複合體進行切割,釋出T4和T3
甲狀腺素(T4 and T3)是甲狀腺濾泡細胞(thyroid epithelial cell|follicular cell)所製造,受腦下腺前葉所釋放的TSH調控。
T4(3,5,3',5'-tetraiodothyronine)是甲狀腺濾泡細胞所製造,由甲狀腺球蛋白所切割而得。
製造過程:
- 鈉-碘幫浦唧出兩個鈉離子,同時唧入一個碘離子,並且碘離子以促進性擴散進入濾泡腔,為一次級主動運輸。
- 甲狀腺氧化酶(Thyroperoxidase)將碘離子( I-)氧化為碘元素( I2),以增加化學活性。
- 甲狀腺氧化酶中碘化了甲狀腺球蛋白的酪胺醯殘基,並且在甲狀腺濾泡細胞的內質網中兩兩結合,並且釋出到膠質中。
- 腦下腺前葉釋出促甲狀腺素(TSH),結合於細胞膜的TSH受器上(一種G蛋白偶聯受體),刺激甲狀腺濾泡細胞對於甲狀腺球蛋白的胞飲作用。
- 攝入的囊泡與甲狀腺濾泡細胞的溶酶體結合,蛋白酶將碘化的球蛋白切割出T4。
- 最後,這些囊泡以胞吐作用釋出甲狀腺素。
甲狀腺素是在酪胺酸的苯環接上碘而成,碘離子與鈉離子經由反向運輸進入濾泡細胞,使細胞內碘離子濃度達到血液中離子濃度的30倍。在甲狀腺過氧化氫酶的作用下,形成單碘酪胺酸(monoiodotyrosine,MIT)以及雙碘酪胺酸(diiodotyrosine,DIT),再結合形成甲狀腺素。
流程:
- DIT + MIT → r-T3 (無生物活性)
- MIT + DIT → T3
- DIT + DIT → T4
周圍 编辑
大多數的甲狀腺素以T4型態存在於血液循環中,並在作用時轉換為活性較強的T3,脫碘酶的缺乏將導致與碘攝取缺乏相同的症狀。
胚胎的甲狀腺素製造 编辑
Thyrotropin-releasing hormone (TRH) is released from hypothalamus by 6 - 8 weeks, and thyroid-stimulating hormone(TSH) secretion from fetal pitutary is evident by 12 weeks of gestation, and fetal production of thyroxine(T4) reaches a clinically significant level at 18–20 weeks.[6] Fetal triiodothyronine (T3) remains low (less than 15 ng/dL) until 30 weeks of gestation, and increases to 50 ng/dL at term.[6] Fetal self-sufficiency of thyroid hormones protects the fetus against e.g. brain development abnormalities caused by maternal hypothyroidism.[7]
碘缺乏對於甲狀腺素合成的影響 编辑
If there is a deficiency of dietary iodine, the thyroid will not be able to make thyroid hormone. The lack of thyroid hormone will lead to decreased negative feedback on the pituitary, leading to increased production ofthyroid-stimulating hormone, which causes the thyroid to enlarge (the resulting medical condition is called endemic colloid goiter; see goiter). This has the effect of increasing the thyroid's ability to trap more iodide, compensating for the iodine deficiency and allowing it to produce adequate amounts of thyroid hormone.
循環及傳訊 编辑
Plasma transport 编辑
Most of the thyroid hormone circulating in the blood is bound to transport proteins. Only a very small fraction of the circulating hormone is free (unbound) and biologically active, hence measuring concentrations of free thyroid hormones is of great diagnostic value.
When thyroid hormone is bound, it is not active, so the amount of free T3/T4 is what is important. For this reason, measuring total thyroxine in the blood can be misleading.
| Type | Percent |
|---|---|
| bound to thyroxine-binding globulin (TBG) | 70% |
| bound to transthyretin or "thyroxine-binding prealbumin" (TTR or TBPA) | 10-15% |
| albumin | 15-20% |
| unbound T4 (fT4) | 0.03% |
| unbound T3 (fT3) | 0.3% |
Despite being lipophilic, T3 and T4 cross the cell membrane via carrier-mediated transport, which is ATP-dependent.[8] The thyroid hormones function via a well-studied set of nuclear receptors in the nucleus of the cell, the thyroid hormone receptors.
T1a and T0a are positively charged and do not cross the membrane; they are believed to function via thetrace amine-associated receptor TAAR1 (TAR1, TA1), a G-protein-coupled receptor located in the cell membrane.
Another critical diagnostic tool is measurement of the amount of thyroid-stimulating hormone (TSH) that is present.
胞外傳訊 编辑
Contrary to common belief, thyroid hormones can not traverse cell membranes in a passive manner like other lipophilicsubstances. The iodine in o-position makes the phenolic OH-group more acidic, resulting in a negative charge at physiological pH. However, at least 10 different active, energy-dependent and genetic-regulated iodothyronine transporters have been identified in humans. They guarantee that intracellular levels of thyroid hormones are higher than in blood plasma or interstitial fluids.[9]
胞內傳訊 编辑
Little is known about intracellular kinetics of thyroid hormones. However, recently it could be demonstrated that thecrystallin CRYM binds 3,5,3′-triiodothyronine in vivo.[10]
Measurement 编辑
Thyroxine and triiodothyronine can be measured as free thyroxine and free triiodothyronine, which are indicators of thyroxine and triiodothyronine activities in the body. They can also be measured as total thyroxine and total triiodothyronine, which also depend on the thyroxine and triiodothyronine that is bound to thyroxine-binding globulin. A related parameter is the free thyroxine index, which is total thyroxine multiplied by thyroid hormone uptake, which, in turn, is a measure of the unbound thyroxine-binding globulins.[11]
甲狀腺的影響 编辑
- Increases cardiac output
- Increases heart rate
- Increases ventilation rate
- Increases basal metabolic rate
- Potentiates the effects of catecholamines (i.e. increases sympathetic activity)
- Potentiates brain development
- Thickens endometrium in females
- increase metabolism of proteins and carbohydrates (i.e. they have a catabolic action[12])
醫學用途 编辑
Both T3 and T4 are used to treat thyroid hormone deficiency (hypothyroidism). They are both absorbed well by the gut, so can be given orally. Levothyroxine is the pharmaceutical name (INN) of levothyroxine sodium (T4), which is metabolised more slowly than T3 and hence usually only needs once-daily administration. Natural desiccated thyroid hormones are derived from pig thyroid glands, and are a "natural" hypothyroid treatment containing 20% T3 and traces of T2, T1and calcitonin. Also available are synthetic combinations of T3/T4 in different ratios (such as liotrix) and pure-T3 medications (INN: liothyronine). Levothyroxine Sodium is usually the first course of treatment tried. Some patients feel they do better on desiccated thyroid hormones; however, this is based on anecdotal evidence and clinical trials have not shown any benefit over the biosynthetic forms.[13]
Thyronamines have no medical usages yet, though their use has been proposed for controlled induction of hypothermia, which causes the brain to enter a protective cycle, useful in preventing damage during ischemic shock.
Synthetic thyroxine was first successfully produced by Charles Robert Harington and George Barger in 1926.
處方 编辑
Today most patients are treated with levothyroxine, or a similar synthetic thyroid hormone.[14][15][16] However, natural thyroid hormone supplements from the dried thyroids of animals are still available.[16] Natural thyroid hormones have become less popular, due to evidence that varying hormone concentrations in the thyroids of animals before they are slaughtered leads to inconsistent potency and stability.[17][18] Levothyroxine contains T4 only and is therefore largely ineffective for patients unable to convert T4 to T3.[19] These patients may choose to take natural thyroid hormone as it contains a mixture of T4 and T3,[16][20][21][22][23] or alternatively supplement with a synthetic T3 treatment.[24] In these cases, synthetic liothyronine is preferred due to the potential differences between drug lots of natural thyroid products. It would be counterintuitive to supplement with T4-only if the patient cannot convert T4 to T3. Some natural thyroid hormone brands are F.D.A. approved, but some are not.[25][26][27] Thyroid hormones are generally well tolerated.[15] Thyroid hormones are usually not dangerous for pregnant women or nursing mothers, but should be given under a doctor's supervision. In fact, if a woman who is hypothyroid is left untreated, her baby is at a higher risk for birth defects. When pregnant, a woman with a low functioning thyroid will also need to increase her dosage of thyroid hormone.[15] One exception is that thyroid hormones may aggravate heart conditions, especially in older patients; therefore, doctors may start these patients on a lower dose & work up to avoid risk of heart attack.[16]
抗甲狀腺素藥物 编辑
Iodine uptake against a concentration gradient is mediated by a sodium-iodine symporter and is linked to a sodium-potassium ATPase. Perchlorate and thiocyanate are drugs that can compete with iodine at this point. Compounds such as goitrin can reduce thyroid hormone production by interfering with iodine oxidation.[28]
相關疾病 编辑
甲狀腺素分泌過多或過少都會導致疾病:
- 甲狀腺機能亢進(Hyperthyroidism):(如凸眼性甲狀腺腫或稱格雷夫斯症Graves Disease),甲狀腺素釋放過多所造成,大約2%的女性及0.2%的男性罹患此病。甲狀腺毒症(Thyrotoxicosis)甲狀腺毒症通常會被甲狀腺機能亢進這個名詞替換,但是彼此之間還是有些許的差異性存在,雖然甲狀腺毒症也與甲狀腺素分泌過多有關,主要原因是攝取過多甲狀腺素的藥片或者是甲狀腺分泌過量,而甲狀腺機能亢進則單指甲狀腺分泌過量而已。
- 甲狀腺機能低下有時會導致精神低落。[29] Some research[30] has shown that T3 is found in the junctions of synapses, and regulates the amounts and activity of serotonin, norepinephrine, and γ-aminobutyric acid (GABA) in the brain.
Preterm births can suffer neurodevelopmental disorders due to lack of maternal thyroid hormones, at a time when their own thyroid is unable to meet their postnatal needs.[31]
参见 编辑
T3和T4 编辑
外部連結 编辑
- Thyroxine bound to proteins in the PDB
甲狀腺疾病的治療
- Thyroid Hormone Treatment Brochure by the American Thyroid Association
- Elaborate article about the use of thyroid drugs Written by an MD
- What is the "Best" Thyroid Drug? Is it Synthroid, Unithroid, Armour, Thyrolar, or Something Else? Article by health activist and patient advocate Mary Shomon
- Thyroid Disease Manager Collection of elaborate medical articles on thyroid disease, including information on thyroid hormones
- Stop the thyroid madness Collection of references to articles comparing different treatment methods of hypothyroidism
參考文獻 编辑
- ^ 參考
- ^ 空引用 (帮助)
- ^ References used in image are found in image article in Commons:Commons:File:Thyroid_systbcvhxcxcfffgem.png#References.
- ^ Chapter 48, "SYNTHESIS OF THYROID HORMONES" in: Walter F., PhD. Boron. Medical Physiology: A Cellular And Molecular Approach. Elsevier/Saunders. 2003: 1300. ISBN 1-4160-2328-3.
- ^ How Iodide Reaches its Site of Utilisation in the Thyroid Gland – Involvement of Solute Carrier 26A4 (Pendrin) and Solute Carrier 5A8 (Apical Iodide Transporter) - a report by Bernard A Rousset. Touch Brieflings 2007
- ^ 6.0 6.1 Page 493 (Table 33-3) in: Eugster, Erica A.; Pescovitz, Ora Hirsch. Pediatric endocrinology: mechanisms, manifestations and management. Hagerstwon, MD: Lippincott Williams & Wilkins. 2004. ISBN 0-7817-4059-2.
- ^ Zoeller RT. Transplacental thyroxine and fetal brain development. J. Clin. Invest. April 2003, 111 (7): 954–7. PMC 152596 . PMID 12671044. doi:10.1172/JCI18236.
- ^ "Plasma Membrane Transport of Thyroid Hormones and Its Role in Thyroid Hormone Metabolism and Bioavailability." Georg Hennemann, Roelof Docter, Edith C. H. Friesema, Marion de Jong, Eric P. Krenning and Theo J. Visser. Endocrine Reviews. August 2001; 22(4):451–476
- ^ Dietrich, J. W., K. Brisseau und B. O. Boehm (2008). "Resorption, Transport und Bioverfügbarkeit von Schilddrüsenhormonen" [Absorption, transport and bio-availability of iodothyronines]. Deutsche Medizinische Wochenschrift 133 (31/21): 1644-8.DOI 10.1055/s-0028-1082780
- ^ Satoru Suzuki, Nobuyoshi Suzuki, Jun-ichirou Mori, Aki Oshima, Shinichi Usami and Kiyoshi Hashizume. μ-Crystallin as an Intracellular 3,5,3′-Triiodothyronine Holder in Vivo. MMolecular Endocrinology April 1, 2007 vol. 21 no. 4 885-894. PMID 17264173
- ^ Military Obstetrics & Gynecology > Thyroid Function Tests In turn citing: Operational Medicine 2001, Health Care in Military Settings, NAVMED P-5139, May 1, 2001, Bureau of Medicine and Surgery, Department of the Navy, 2300 E Street NW, Washington, D.C., 20372-5300
- ^ http://www.ncbi.nlm.nih.gov/pubmed/2884552
- ^ "Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials." Grozinsky-Glasberg S; Fraser A; Nahshoni E; Weizman A; Leibovici L. J Clin Endocrinol Metab. 2006 Jul;91(7):2592-
- ^ Robert Lloyd Segal, MD Endocrinologist
- ^ 15.0 15.1 15.2 "preferred thyroid hormone --Levothyroxine Sodium (Synthroid, Levoxyl, Levothroid, Unithroid)", Retrieved on 2009-3-27
- ^ 16.0 16.1 16.2 16.3 "Hypothyroidism Causes, Symptoms, Diagnosis, Treatment Information Produced by Medical Doctors", Retrieved on 2009-3-27
- ^ Cooper, DS. Thyroid hormone treatment: new insights into an old therapy.. JAMA. 1989, 261 (18): 2694–2695. doi:10.1001/jama.1989.03420180118042.
- ^ Clyde, PW; Harari AE, Mohamed Shakir KM. Synthetic Thyroxine vs Desiccated Thyroid -Reply (citing Cooper, DS, above). JAMA. 2004, 291 (12): 1445. doi:10.1001/jama.291.12.1445-b.
- ^ Thyroid hormone replacement therapy
- ^ "Consequences of Not Taking Thyroid Medications -Implications of Failing to Take Prescription Thyroid Drugs", Retrieved on 2009-3-27
- ^ "Armour Thyroid", Retrieved on 4-1-2009
- ^ "Nature-Throid", Retrieved on 4-1-2009
- ^ "Armour Thyroid Shortages Worsening: What Can Thyroid Patients Do?", Retrieved on 2009-3-27
- ^ Liothyronine
- ^ "Thyroid Information", Retrieved on 2009-3-27
- ^ "Desiccated thyroid in a nutritional supplement | Journal of Family Practice | Find Articles at BNET", Retrieved on 2009-3-27
- ^ "Nature-Throid", Retrieved on 4-1-2009
- ^ Spiegel C, Bestetti GE, Rossi GL, Blum JW. Normal circulating triiodothyronine concentrations are maintained despite severe hypothyroidism in growing pigs fed rapeseed presscake meal. J. Nutr. September 1993, 123 (9): 1554–61. PMID 8360780.
- ^ Kirkegaard C, Faber J. The role of thyroid hormones in depression. Eur J Endocrinol. 1998, 138 (1): 1–9. PMID 9461307. doi:10.1530/eje.0.1380001.
- ^ Dratman M, Gordon J. Thyroid hormones as neurotransmitters. Thyroid. 1996, 6 (6): 639–47. PMID 9001201. doi:10.1089/thy.1996.6.639.
- ^ Berbel P, Navarro D, Ausó E, Varea E, Rodríguez AE, Ballesta JJ, Salinas M, Flores E, Faura CC, de Escobar GM. (2010).Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity. Cereb Cortex. 20(6):1462-75. PMID 19812240.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||



