吡维铵
化合物
吡维铵(英语:Pyrvinium)是一种对蛲虫有效的驱虫药。[1]吡维铵已几种方式存在,例如吡维氯铵、对甲苯磺酸吡维铵、三氟甲磺酸吡维铵和双羟萘酸吡维铵(或叫帕莫酸吡维铵及恩波酸吡维铵)。[2][3]吡维铵被鉴定为一种有效的Wnt抑制剂,通过激活酪蛋白激酶CK1α发挥作用。[4][5]
 | |
| 臨床資料 | |
|---|---|
| AHFS/Drugs.com | Micromedex详细消费者药物信息 |
| ATC碼 | |
| 识别信息 | |
| |
| CAS号 | 7187-62-4 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.543 |
| 化学信息 | |
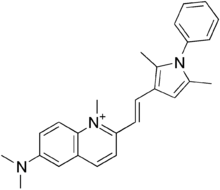
| 化学式 | C26H28N3+ |
| 摩尔质量 | 382.53 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
合成
编辑其中一种合成方法基于斯克劳普合成和帕尔-克诺尔合成。[6]最近,报道了通过弗里德兰德喹啉合成替代三氟甲磺酸吡维铵的收敛合成策略。[3]
参考资料
编辑- ^ Desai AS. Single-dose treatment of oxyuriasis with pyrvinium embonate. British Medical Journal. December 1962, 2 (5319): 1583–5. PMC 1926864 . PMID 14027194. doi:10.1136/bmj.2.5319.1583.
- ^ Pyrvinium. PubChem. U.S. National Library of Medicine. [2023-02-06]. (原始内容存档于2016-04-02).
- ^ 3.0 3.1 Mao Y, Lin N, Tian W, Huang Z. New Synthesis of Pyrvinium That inhibits the β-Catenin/Tcf4 Pathway. Heterocycles. 2012, 85 (5): 1179–1185. doi:10.3987/COM-12-12446.
- ^ Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLOS ONE. 2010, 5 (11): e15521. Bibcode:2010PLoSO...515521S. PMC 2993965 . PMID 21170416. doi:10.1371/journal.pone.0015521 .
- ^ Shen C, Nayak A, Melendez RA, Robbins DJ. Casein Kinase 1α as a Regulator of Wnt-Driven Cancer. International Journal of Molecular Sciences. 2020, 21 (16): 5940. PMC 7460588 . PMID 32824859. doi:10.3390/ijms21165940 .
- ^ 6.0 6.1 WO 2006078754,Macdonald JE, Hysell MK, Yu D, Li H, Wong-Staal F,「Novel Quinolinium Salts and Derivatives」,发表于2006-07-27
- ^ Esumi H, Lu J, Kurashima Y, Hanaoka T. Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl]-1-methyl-quinolinium pamoate salt, showing preferential cytotoxicity during glucose starvation. Cancer Science. August 2004, 95 (8): 685–90. PMID 15298733. doi:10.1111/j.1349-7006.2004.tb03330.x .