高良姜素
化合物
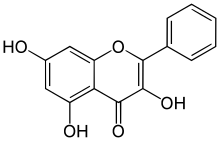
高良姜素(化学式C15H10O5)是一种黄酮醇。
| 高良姜素 | |
|---|---|
| |
| |
| IUPAC名 3,5,7-Trihydroxy-2-phenylchromen-4-one | |
| 英文名 | Galangin |
| 别名 | 3,5,7-三羟基黄酮 |
| 识别 | |
| CAS号 | 548-83-4 |
| PubChem | 5281616 |
| ChemSpider | 4444935 |
| SMILES |
|
| InChI |
|
| InChIKey | VCCRNZQBSJXYJD-UHFFFAOYAC |
| ChEBI | 5262 |
| KEGG | C10044 |
| IUPHAR配体 | 410 |
| 性质 | |
| 化学式 | C15H10O5 |
| 摩尔质量 | 270.24 g·mol−1 |
| 密度 | 1.579 g/mL |
| 熔点 | 214-215 °C(487-488 K) |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
来源
编辑生物活性
编辑研究发现,高良姜素在体外具有抗菌[5][6]和抗病毒活性。[7]同时,体外试验还发现高良姜素能抑制乳腺癌细胞的增殖。[8][9]
参考资料
编辑- ^ Ciolino, H. P.; Yeh, G. C. The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor. British Journal of Cancer. 1999, 79 (9/10): 1340–1346. PMC 2362711 . PMID 10188874. doi:10.1038/sj.bjc.6690216.
- ^ Afolayan AJ, Meyer JJ. The antimicrobial activity of 3,5,7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens. Journal of Ethnopharmacology. 1997, 57 (3): 177–181. PMID 9292410. doi:10.1016/s0378-8741(97)00065-2.
- ^ Kaur, A.; Singh, R.; Dey, C. S.; Sharma, S. S.; Bhutani, K. K.; Singh, I. P. Antileishmanial phenylpropanoids from Alpinia galanga (Linn.) Willd (PDF). Indian Journal of Experimental Biology. 2010, 48 (3): 314–317 [2021-04-10]. PMID 21046987. (原始内容存档 (PDF)于2020-01-25).
- ^ Tosi, E; Re, E; Ortega, M; Cazzoli, A. Food preservative based on propolis: Bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia coli. Food Chemistry. 2007, 104: 1025. doi:10.1016/j.foodchem.2007.01.011.
- ^ Cushnie TP, Lamb AJ. Assessment of the antibacterial activity of galangin against 4-quinolone resistant strains of Staphylococcus aureus. Phytomedicine. 2006, 13 (3): 187–191. PMID 16428027. doi:10.1016/j.phymed.2004.07.003.
- ^ Cushnie TP, Lamb AJ. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. Journal of Ethnopharmacology. 2005, 101 (1–3): 243–248. PMID 15985350. doi:10.1016/j.jep.2005.04.014.
- ^ Afolayan AJ, Meyer JJ, Taylor MB, Erasmus D. Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens. Journal of Ethnopharmacology. 1997, 56 (2): 165–169. PMID 917497. doi:10.1016/s0378-8741(97)01514-6.
- ^ So, F. V.; Guthrie, N.; Chambers, A. F.; Moussa, M.; Carroll, K. K. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutrition and Cancer. 1996, 26 (2): 167–181. PMID 8875554. doi:10.1080/01635589609514473.
- ^ So, F.; Guthrie, N.; Chambers, A. F.; Carroll, K. K. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer Letters. 1997, 112 (2): 127–133. PMID 9066718. doi:10.1016/S0304-3835(96)04557-0.