硼烯
硼烯是硼原子的單分子層結晶,也就是說它是硼的二維同素異形體。硼烯最早在1990年代中期被理論預言,[1]2015年在實驗中不同結構的硼烯被證實存在。[2][3]
性質 編輯
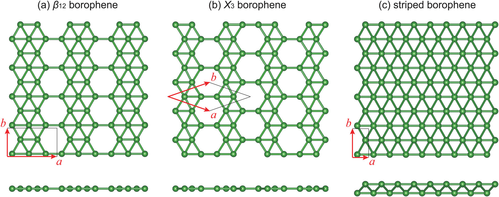
在超高真空條件下的潔淨金屬表面上,不同種類的硼烯晶體和金屬可以以原子尺度的薄片形式製備。[2][3]如圖1所示,它們的原子結構由三角和六邊形圖樣混合而成。其原子結構是平面內雙中心和多中心的成鍵的結果,這對於硼這樣的缺電子元素來說非常典型。[4]
硼烯在面內有具有彈性和理想的強度。在某些情況下它可以比石墨烯更堅固且更柔韌。[5]例如,硼納米管比其他所有已知的碳和非碳的納米結構都有更高的二維楊氏模量。[6]由於硼烯面內多中心鍵合的流變性,面內的拉伸載荷下會使得硼烷發生獨特的結構相變。[7]理論上硼烯有着更高的比熱容,電導率和離子傳輸特性,它有作為電池陽極材料的潛力。氫很容易吸附到硼苯上,使它具有作為儲氫材料的潛力——可以儲存其重量15%以上的氫。硼烯可以催化氫分子分解成氫離子,並催化分解水。
歷史 編輯
36簇可能是最小的硼烯;前視圖和側視圖
I. Boustani和A. Quandt的計算研究表明,小的硼團簇不像硼烷那樣是正二十面體,相反它們被發現是准平面的(見圖2)。[1]這導致了所謂的Aufbau原理被發現[8] ,該原理預測了硼烯、硼富勒烯(硼球烯)[9]和硼納米管存在的可能性。[10][11][12]
進一步的研究表明,擴展的三角形硼烯(圖1(c))是金屬性的,並有着非平面的彎曲的幾何形狀。[13][14]進一步的從穩定B80硼富勒烯開始的計算[15]表明,有着蜂窩結構並部分由六邊形孔填充的擴展的片狀硼烯是穩定的。[16][17]這些硼烯的結構被預言是金屬性的。所謂的γ片(β12硼烯或 υ1/6 片)示於圖1(a)。
硼團簇的平面性首先由王來生的研究小組通過實驗證實。[18]晚些時候他們證明了B
36結構(見圖2)是具有六重對稱性和完美六邊形空缺的最小硼團簇,它可能可以作為擴展二維硼烯的基礎。[19]
在硅烯被製備後,多個研究小組預測,硼烯有可能能在金屬表面上被製取。[20][21][22]特別地,硼烯的晶格結構取決於金屬表面,顯示出不同於自由狀態下的晶格結構。[23]
在2015年,兩個研究小組成功地在超高真空條件下在銀(111)表面合成了不同的硼烯的相。[2][3]在三個製取成功的硼烯的相中(見圖1),υ1/6 片或 β12在早先的理論中被證明是銀(111)面上的基態,[23]而χ3硼烯是Xiao Chen Zeng團隊在2012年預測的。[24]目前為止硼烯僅存在於底物上,對把它們轉移到與設備兼容的襯底上的方法的研究是有必要的,但也是一個挑戰。[25]
在理論計算的支持下,原子尺度的表徵揭示的結構讓人想起混合着的包含三角和六方結構的硼團簇,正如先前的理論預言的那樣,如圖1所示。掃描隧道顯微鏡證實硼烯是金屬性的。這與硼的同素異形體不同——後者是半導體並具有基於B12二十面體的原子結構。[ 需要引用 ]
參見 編輯
參考文獻 編輯
- ^ 1.0 1.1 Boustani, Ihsan. New quasi-planar surfaces of bare boron. Surface Science. January 1997, 370 (2–3): 355–363. Bibcode:1997SurSc.370..355B. doi:10.1016/S0039-6028(96)00969-7.
- ^ 2.0 2.1 2.2 Mannix, A. J.; Zhou, X.-F.; Kiraly, B.; Wood, J. D.; Alducin, D.; Myers, B. D.; Liu, X.; Fisher, B. L.; Santiago, U. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science. 17 December 2015, 350 (6267): 1513–1516. Bibcode:2015Sci...350.1513M. PMC 4922135 . PMID 26680195. doi:10.1126/science.aad1080.
- ^ 3.0 3.1 3.2 Feng, Baojie; Zhang, Jin; Zhong, Qing; Li, Wenbin; Li, Shuai; Li, Hui; Cheng, Peng; Meng, Sheng; Chen, Lan. Experimental realization of two-dimensional boron sheets. Nature Chemistry. 28 March 2016, 8 (6): 563–568. Bibcode:2016NatCh...8..563F. PMID 27219700. arXiv:1512.05029 . doi:10.1038/nchem.2491.
- ^ Pauling, Linus. The nature of the chemical bond 3rd. Cornell University Press. 1960. ISBN 0-8014-0333-2.
- ^ arXiv, Emerging Technology from the. Sorry, graphene—borophene is the new wonder material that's got everyone excited. MIT Technology Review. [2019-08-02]. (原始內容存檔於2019-10-14) (美國英語).
- ^ Kochaev, A. Elastic properties of noncarbon nanotubes as compared to carbon nanotubes. Physical Review B. 11 October 2017, 96 (15): 155428. doi:10.1103/PhysRevB.96.155428.
- ^ Zhang, Z.; Yang, Yang.; Penev, E.S.; Yakobson, B.I. Elasticity, Flexibility, and Ideal Strength of Borophenes. Advanced Functional Materials. 11 January 2017, 27 (9): 1605059. arXiv:1609.07533 . doi:10.1002/adfm.201605059.
- ^ Boustani, Ihsan. Systematic ab initio investigation of bare boron clusters: Determination of the geometry and electronic structures of Bn (n=2-14). Physical Review B. 15 June 1997, 55 (24): 16426–16438. doi:10.1103/PhysRevB.55.16426.
- ^ Boustani, Ihsan. New Convex and Spherical Structures of Bare Boron Clusters. Journal of Solid State Chemistry. October 1997, 133 (1): 182–189. doi:10.1006/jssc.1997.7424.
- ^ Boustani, I; Quandt, A. Nanotubules of bare boron clusters: Ab initio and density functional study. Europhysics Letters (EPL). 1 September 1997, 39 (5): 527–532. doi:10.1209/epl/i1997-00388-9.
- ^ Gindulytė, Asta; Lipscomb, William N.; Massa, Lou. Proposed Boron Nanotubes. Inorganic Chemistry. December 1998, 37 (25): 6544–6545. PMID 11670779. doi:10.1021/ic980559o.
- ^ Quandt, Alexander; Boustani, Ihsan. Boron Nanotubes. ChemPhysChem. 14 October 2005, 6 (10): 2001–2008. PMID 16208735. doi:10.1002/cphc.200500205.
- ^ Boustani, Ihsan; Quandt, Alexander; Hernández, Eduardo; Rubio, Angel. New boron based nanostructured materials. The Journal of Chemical Physics. 8 February 1999, 110 (6): 3176–3185. doi:10.1063/1.477976.
- ^ Kunstmann, Jens; Quandt, Alexander. Broad boron sheets and boron nanotubes: An ab initio study of structural, electronic, and mechanical properties. Physical Review B. 12 July 2006, 74 (3): 035413. arXiv:cond-mat/0509455 . doi:10.1103/PhysRevB.74.035413.
- ^ Gonzalez Szwacki, Nevill; Sadrzadeh, Arta; Yakobson, Boris I. B80 Fullerene: An Ab Initio Prediction of Geometry, Stability, and Electronic Structure. Physical Review Letters. 20 April 2007, 98 (16): 166804. Bibcode:2007PhRvL..98p6804G. PMID 17501448. doi:10.1103/PhysRevLett.98.166804.
- ^ Tang, Hui & Ismail-Beigi, Sohrab. Novel Precursors for Boron Nanotubes: The Competition of Two-Center and Three-Center Bonding in Boron Sheets. Physical Review Letters. 2007, 99 (11): 115501. Bibcode:2007PhRvL..99k5501T. PMID 17930448. arXiv:0710.0593 . doi:10.1103/PhysRevLett.99.115501.
- ^ Özdoğan, C.; Mukhopadhyay, S.; Hayami, W.; Güvenç, Z. B.; Pandey, R.; Boustani, I. The Unusually Stable B100 Fullerene, Structural Transitions in Boron Nanostructures, and a Comparative Study of α- and γ-Boron and Sheets. The Journal of Physical Chemistry C. 18 March 2010, 114 (10): 4362–4375. doi:10.1021/jp911641u.
- ^ Zhai, Hua-Jin; Kiran, Boggavarapu; Li, Jun; Wang, Lai-Sheng. Hydrocarbon analogues of boron clusters — planarity, aromaticity and antiaromaticity. Nature Materials. 9 November 2003, 2 (12): 827–833. PMID 14608377. doi:10.1038/nmat1012.
- ^ Piazza, Z. A.; Hu, H. S.; Li, W. L.; Zhao, Y. F.; Li, J.; Wang, L. S. Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets. Nature Communications. 2014, 5: 3113. Bibcode:2014NatCo...5.3113P. PMID 24445427. doi:10.1038/ncomms4113.
- ^ Zhang, L. Z.; Yan, Q. B.; Du, S. X.; Su, G.; Gao, H.-J. Boron Sheet Adsorbed on Metal Surfaces: Structures and Electronic Properties. The Journal of Physical Chemistry C. 15 August 2012, 116 (34): 18202–18206. doi:10.1021/jp303616d.
- ^ Liu, Yuanyue; Penev, Evgeni S.; Yakobson, Boris I. Probing the Synthesis of Two-Dimensional Boron by First-Principles Computations. Angewandte Chemie International Edition. 11 March 2013, 52 (11): 3156–3159. PMID 23355180. arXiv:1312.0656 . doi:10.1002/anie.201207972.
- ^ Liu, Hongsheng; Gao, Junfeng; Zhao, Jijun. From Boron Cluster to Two-Dimensional Boron Sheet on Cu(111) Surface: Growth Mechanism and Hole Formation. Scientific Reports. 18 November 2013, 3 (1): 3238. PMC 3831238 . PMID 24241341. doi:10.1038/srep03238.
- ^ 23.0 23.1 Zhang, Z.; Yang, Y.; Gao, G.; Yakobson, B.I. Two-Dimensional Boron Monolayers Mediated by Metal Substrates. Angewandte Chemie International Edition. 2 September 2015, 54 (44): 13022–13026. PMID 26331848. doi:10.1002/anie.201505425.
- ^ Wu, Xiaojun; Dai, Jun; Zhao, Yu; Zhu, Zhiwen; Yang, Jinlong; Zeng, Xiao Cheng. Two-Dimensional Boron Monolayer Sheets. ACS Nano. 20 July 2012, 6 (8): 7443–7453. PMID 22816319. doi:10.1021/nn302696v.
- ^ Zhang, Z.; Penev, E.S.; Yakobson, B.I. Two-dimensional boron: structures, properties and applications. Chemical Society Reviews. 31 October 2017, 46 (22): 6746–6763. PMID 29085946. doi:10.1039/c7cs00261k.