2-氟-6-氯甲苯
化合物
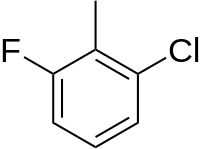
2-氟-6-氯甲苯是一种有机化合物,化学式为C7H6ClF。它可由3-氯-2-甲基苯胺、亚硝酸钠和氢氟酸为原料反应得到。[2]在铁存在下,它和溴反应,可以得到2-氯-3-溴-6-氟甲苯。[3]在过氧化苯甲酰存在下,它和N-溴代丁二酰亚胺反应,可以得到2-氟-6-氯-α,α-二溴甲苯。[4]
| 2-氟-6-氯甲苯 | |
|---|---|
| |
| IUPAC名 1-chloro-3-fluoro-2-methylbenzene | |
| 别名 | 2-氯-6-氟甲苯 |
| 识别 | |
| CAS号 | 443-83-4 |
| PubChem | 9933 |
| SMILES |
|
| InChI |
|
| EINECS | 207-141-9 |
| 性质 | |
| 化学式 | C7H6ClF |
| 摩尔质量 | 144.57 g·mol−1 |
| 沸点 | 154 °C[1] |
| 危险性 | |
GHS危险性符号 
| |
| GHS提示词 | 警告 |
| H-术语 | H226, H302, H312, H315, H332, H335 |
| P-术语 | P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P302+352, P303+361+353, P304+340 |
| 闪点 | 46 °C (115 °F) |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
编辑- ^ "PhysProp" data were obtained from Syracuse Research Corporation of Syracuse, New York (US). Retrieved from SciFinder. [2022-3-19].
- ^ Li, Xiao Yun; Zheng, Qiong; Yu, Peng; Huang, Chi; Jian, Li Duan; Peng, Jun Qing. A new synthetic route for preparation of 2-chloro-6-fluorobenzonitrile and 2-chloro-6-fluorobenzoic acid. Chinese Chemical Letters, 2004. 15 (8): 895-898. ISSN 1001-8417.
- ^ Nathalie Schlienger, Birgitte W. Lund, Jan Pawlas, Fabrizio Badalassi, Fabio Bertozzi, Rasmus Lewinsky, Alma Fejzic, Mikkel B. Thygesen, Ali Tabatabaei, Stefania Risso Bradley, Luis R. Gardell, Fabrice Piu, Roger Olsson. Synthesis, Structure−Activity Relationships, and Characterization of Novel Nonsteroidal and Selective Androgen Receptor Modulators. Journal of Medicinal Chemistry. 2009-11-26, 52 (22): 7186–7191 [2022-03-19]. ISSN 0022-2623. doi:10.1021/jm901149c. (原始内容存档于2021-12-25) (英语).
- ^ Kambappa Vinaya, Ganganahalli K. Chandrashekara, Prasanna D. Shivaramu. One-pot synthesis of 3,5-diaryl substituted-1,2,4-oxadiazoles using gem -dibromomethylarenes. Canadian Journal of Chemistry. 2019-09, 97 (9): 690–696 [2022-03-19]. ISSN 0008-4042. doi:10.1139/cjc-2018-0333 (英语).