原肠胚形成
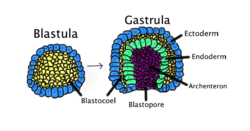
原肠胚形成(Gastrulation)是大部分动物胚胎发育中都会经历的一个阶段。在本阶段中,只有一层细胞的囊胚会发生重组,形成一个含有三个胚层(即外胚层(ectoderm)、中胚层(mesoderm)、内胚层(endoderm))细胞的原肠胚(gastrula)[1][2]。
| 原肠胚形成 | |
|---|---|
 | |
| 标识字符 | |
| MeSH | D054262 |
| 格雷氏 | p.47 |
| 《解剖学术语》 [在维基数据上编辑] | |
原肠胚形成发生于卵裂之后,原肠胚形成完成后,胚胎进入原肠胚时期,开始器官发生过程。新形成的三个胚层的细胞会组合并发育为器官[3]。每一个胚层的细胞都能发育为特定的器官和组织。外胚层会发育为表皮、神经嵴,以及之后会发育为神经系统的组织。中胚层细胞位于外胚层细胞和内胚层细胞之间,能发育为体节,生成肌肉,以及属于肋骨、椎骨的软骨。另外,中胚层还能发育为真皮、脊髓、血管与血液、骨,以及结缔组织。内胚层细胞则会发育为消化系统和呼吸系统的上皮,比如肝和胰腺[4]。原肠胚形成过程结束后,机体的细胞都会组织为被上皮等缔合的细胞包围的群体,或者像间充质那样分散在各处[2][5]。
原肠胚形成的分子机制以及所需时间在不同的生物中是不同的。不过,不同生物之间的原肠胚形成仍然有一些共同点,如:一,胚胎的拓扑结构会发生变化,从单连通的表面(类似于球面)变为非单连通的表面(类似于环面)。二,胚胎细胞会分化为外胚层细胞、中胚层细胞、内胚层细胞(部分低等生物无中胚层细胞)。三,内胚层细胞会出现消化功能[6]。另外,尽管动物原肠胚形成的具体模式千差万别,但总的来说原肠胚形成过程中,细胞的移动可以归纳为五种:内陷(Invagination)、内卷(Involution)、内移(Ingression)、分层(delamination)、外包(epiboly)[7]。
“原肠胚”(gastrula)以及“原肠胚形成”(gastrulation)这两个名词都是由恩斯特·海克尔(Ernst Haeckel)在他发表于1872年的著作《钙质海绵生物学》(Biology of Calcareous Sponges)中首次提出的。刘易斯·沃伯特(Lewis Wolpert),发育生物学的先驱之一,曾这样说:“出生、结婚、死亡都不是你一生中最重要的时刻,原肠胚形成才是。”[8]。
体内(羊膜上)
编辑总览
编辑在羊膜(爬行类、鸟类、哺乳动物)上,原肠胚形成与原肠腔(archenteron)上出现的一个开口,胚孔的出现有密切关系。注意胚孔并不是囊胚阶段上囊胚腔上的开口,而是一个新的开口。胚孔能推动囊胚表面的细胞聚合在一起。在羊膜上,原肠胚形成可以分为以下几个大步骤:一,胚胎变为不对称;二,原条形成;三,原条表皮细胞发生上皮-间充质转换。同时,原条区域细胞发生内移,形成胚层[4]。
原口动物(protostome)和后口动物(deuterostome)口肛发生的差异在于,原口动物口的发生先于肛门,胚孔会发育为口。而后口动物口的发生后于肛门,胚孔会发育为肛门。它们的英文名也正是据此而来:原口动物(protostome)的英文名来自两个希腊语单词:“πρώτος + στόμα”,意思分别为“先”和“口”。后口动物(deuterostom)的英文名同样来自两个希腊单词:“δεύτερος + στόμα”,意思分别是“第二”(次)和“口”。
对称性的失去
编辑在原肠胚形成的准备阶段,胚胎会形成远近轴(proximal-distal axis)以及前后轴(anterior-posterior axis),不对称性也随这两个轴的发生而出现。胚胎的卵圆筒形成标志着远近轴的形成:近端的胚胎外组织会形成胎盘样组织,而远端则是上胚层。骨塑型蛋白(BMP)、成纤维细胞生长因子(FGF)、Nodal、Wnt等信号分子介导的信号转导途径参与了这一过程。内脏内胚层包围表皮。远端内脏内胚层(DVE)会迁移到胚胎的前部,形成前内脏内胚层(AVE),打破前后对称性。上述过程受Nodal信号通路的调控[4]。
原条的产生
编辑原条在原肠胚形成的初始阶段产生。原条位于后侧胚胎外组织以及表皮之间的连结处以及内移发生的区域[9]。原条的形成与科氏镰区域的细胞中的NODAL信号通路以及由胚胎外组织激活的BMP4信号通路有很大关系[4][9][10]。Cer1和Lefty1通过拮抗Nodal信号通路,能将原条形成限制在特定区域[11]。原条区域(在形成后)会继续往远端生长[4]。
During the early stages of development, the primitive streak is the structure that will establish bilateral symmetry, determine the site of gastrulation and initiate germ layer formation. To form the streak, reptiles, birds and mammals arrange mesenchymal cells along the prospective midline, establishing the first embryonic axis, as well as the place where cells will ingress and migrate during the process of gastrulation and germ layer formation.[12] The primitive streak extends through this midline and creates the antero-posterior body axis,[13] becoming the first symmetry-breaking event in the embryo, and marks the beginning of gastrulation.[14] This process involves the ingression of mesoderm and endoderm progenitors and their migration to their ultimate position,[13][15] where they will differentiate into the three germ layers.[12] The localization of the cell adhesion and signaling molecule beta-catenin is critical to the proper formation of the organizer region that is responsible for initiating gastrulation.
上皮-间充质转换与内移
编辑In order for the cells to move from the epithelium of the epiblast through the primitive streak to form a new layer, the cells must undergo an epithelial to mesenchymal transition (EMT) to lose their epithelial characteristics, such as cell-cell adhesion. FGF signaling is necessary for proper EMT. FGFR1 is needed for the up regulation of SNAI1, which down regulates E-cadherin, causing a loss of cell adhesion. Following the EMT, the cells ingress through the primitive streak and spread out to form a new layer of cells or join existing layers. FGF8 is implicated in the process of this dispersal from the primitive streak.[11]
体外原肠胚形成
编辑许多科学家都在尝试通过体外实验的方法与胚胎实验进行对比对照,以更加深入的研究原肠胚形成的过程。通常,研究人员会使用2D(传统)或[16][17][18]3D(培养拟原肠胚(Gastruloid)的细胞培养方法[19][20]培养胚胎干细胞(ESC)或人工诱导性多能干细胞(iPSC)。基于多种组织培养方法的体外培养方法相比体内实验的方法有成本低、符合3R原则、能以空间和时间特异性的方式准确地使用激动剂/拮抗剂等优势(在体内的原肠胚发生时期就很难做到第三点)。不过,体外实验多少会和体内实验存在差异,因而与体内的胚胎发育进行对比是必要的[20]。
To illustrate this, the guided differentiation of mouse ESCs has resulted in generating primitive streak-like cells that display many of the characteristics of epiblast cells that traverse through the primitive streak[16] (e.g. transient brachyury up regulation and the cellular changes associated with an epithelial to mesenchymal transition[16]), and human ESCs cultured on micro patterns, treated with BMP4, can generate spatial differentiation pattern similar to the arrangement of the germ layers in the human embryo.[17][18] Finally, using 3D embryoid body- and organoid-based techniques, small aggregates of mouse ESCs (Embryonic Organoids, or Gastruloids) are able to show a number of processes of early mammalian embryo development such as symmetry-breaking, polarisation of gene expression, gastrulation-like movements, axial elongation and the generation of all three embryonic axes (anteroposterior, dorsoventral and left-right axes).[19][20]
参见
编辑参考
编辑- ^ Mundlos 2009: p. 422 (页面存档备份,存于互联网档案馆)
- ^ 2.0 2.1 McGeady, 2004: p. 34
- ^ Hall, 1998: pp. 132-134 (页面存档备份,存于互联网档案馆)
- ^ 4.0 4.1 4.2 4.3 4.4 Arnold & Robinson, 2009
- ^ Hall, 1998: p. 177 (页面存档备份,存于互联网档案馆)
- ^ Harrison 2011: p. 206 (页面存档备份,存于互联网档案馆)
- ^ Gilbert 2010: p. 164.
- ^ Ereskovsky 2010: p. 236 (页面存档备份,存于互联网档案馆)
- ^ 9.0 9.1 Tam & Behringer, 1997
- ^ Catala, 2005: p. 1535 (页面存档备份,存于互联网档案馆)
- ^ 11.0 11.1 Tam, P.P.; Loebel, D.A. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007, 8 (5): 368–81. PMID 17387317. doi:10.1038/nrg2084.
- ^ 12.0 12.1 Mikawa T, Poh AM, Kelly KA, Ishii Y, Reese DE. Induction and patterning of the primitive streak, an organizing center of gastrulation in the amniote.. Dev Dyn. 2004, 229 (3): 422–32. PMID 14991697. doi:10.1002/dvdy.10458.
- ^ 13.0 13.1 Downs KM. The enigmatic primitive streak: prevailing notions and challenges concerning the body axis of mammals.. BioEssays. 2009, 31 (8): 892–902. PMC 2949267 . PMID 19609969. doi:10.1002/bies.200900038.
- ^ Chuai M, Zeng W, Yang X, Boychenko V, Glazier JA, Weijer CJ. Cell movement during chick primitive streak formation.. Dev Biol. 2006,. 296(1)) (1): 137–49. PMC 2556955 . PMID 16725136. doi:10.1016/j.ydbio.2006.04.451.
- ^ Chuai M, Weijer CJ. The mechanisms underlying primitive streak formation in the chick embryo.. Curr Top Dev Biol. 2008, 81: 135–56. PMID 18023726. doi:10.1016/S0070-2153(07)81004-0.
- ^ 16.0 16.1 16.2 Turner, David A.; Rué, Pau; Mackenzie, Jonathan P.; Davies, Eleanor; Martinez Arias, Alfonso. Brachyury cooperates with Wnt/β-catenin signalling to elicit primitive-streak-like behaviour in differentiating mouse embryonic stem cells. BMC Biology. 2014-01-01, 12: 63. ISSN 1741-7007. PMC 4171571 . PMID 25115237. doi:10.1186/s12915-014-0063-7.
- ^ 17.0 17.1 Warmflash, Aryeh; Sorre, Benoit; Etoc, Fred; Siggia, Eric D; Brivanlou, Ali H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nature Methods: 847–854. PMC 4341966 . PMID 24973948. doi:10.1038/nmeth.3016.
- ^ 18.0 18.1 Etoc, Fred; Metzger, Jakob; Ruzo, Albert; Kirst, Christoph; Yoney, Anna; Ozair, M. Zeeshan; Brivanlou, Ali H.; Siggia, Eric D. A Balance between Secreted Inhibitors and Edge Sensing Controls Gastruloid Self-Organization. Developmental Cell: 302–315. [2017-01-27]. doi:10.1016/j.devcel.2016.09.016. (原始内容存档于2020-08-15).
- ^ 19.0 19.1 Brink, Susanne C. van den; Baillie-Johnson, Peter; Balayo, Tina; Hadjantonakis, Anna-Katerina; Nowotschin, Sonja; Turner, David A.; Arias, Alfonso Martinez. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development. 2014-11-15, 141 (22): 4231–4242 [2017-01-27]. ISSN 0950-1991. PMC 4302915 . PMID 25371360. doi:10.1242/dev.113001. (原始内容存档于2020-06-12) (英语).
- ^ 20.0 20.1 20.2 Turner, David Andrew; Glodowski, Cherise R.; Luz, Alonso-Crisostomo; Baillie-Johnson, Peter; Hayward, Penny C.; Collignon, Jérôme; Gustavsen, Carsten; Serup, Palle; Schröter, Christian. Interactions between Nodal and Wnt signalling Drive Robust Symmetry Breaking and Axial Organisation in Gastruloids (Embryonic Organoids). bioRxiv. 2016-05-13: 051722 [2017-01-27]. doi:10.1101/051722. (原始内容存档于2018-06-04) (英语).
参考书目
编辑- Arnold, Sebastian J.; Robertson, Elizabeth J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol. 2009, 10 (2): 91–103. PMID 19129791. doi:10.1038/nrm2618.
- Catala, Martin. Embryology of the Spine and Spinal Cord. Tortori-Donati, Paolo et al (编). Pediatric Neuroradiology: Brain. Springer. 2005. ISBN 978-3-540-41077-5.
- Ereskovsky, Alexander V. The Comparative Embryology of Sponges. Springer. 2010. ISBN 978-90-481-8574-0.
- Gilbert, Scott F. Developmental Biology Ninth. Sinauer Associates. 2010. ISBN 978-0-87893-558-1.
- Hall, Brian Keith. 8.3.3 The gastrula and gastrulation. Evolutionary developmental biology 2nd. The Netherlands: Kluwer Academic Publishers. 1998. ISBN 978-0-412-78580-1.
- Harrison, Lionel G. The Shaping of Life: The Generation of Biological Pattern. Cambridge University Press. 2011. ISBN 978-0-521-55350-6.
- McGeady, Thomas A. (编). Gastrulation. Veterinary embryology. Wiley-Blackwell. 2006 [2017-01-27]. ISBN 978-1-4051-1147-8. (原始内容存档于2020-07-02).
- Mundlos, Stefan. Gene action: developmental genetics. Speicher, Michael et al (编). Vogel and Motulsky's Human Genetics: Problems and Approaches 4th. Springer. 2009. ISBN 978-3-540-37653-8. doi:10.1007/978-3-540-37654-5.
- Tam, Patrick P.L.; Behringer, Richard R. Mouse gastrulation: the formation of a mammalian body plan. Mech. Dev. 1997, 68 (1-2): 3–25. PMID 9431800. doi:10.1016/S0925-4773(97)00123-8.
拓展阅读
编辑- Baron, Margaret H. Embryonic Induction of Mammalian Hematopoiesis and Vasculogenesis. Zon, Leonard I. (编). Hematopoiesis: a developmental approach. Oxford University Press. 2001 [2017-01-27]. ISBN 978-0-19-512450-7. (原始内容存档于2019-05-14).
- Cullen, K.E. embryology and early animal development. Encyclopedia of life science, Volume 2. Infobase. 2009. ISBN 978-0-8160-7008-4.
- Forgács, G. & Newman, Stuart A. Cleavage and blastula formation. Biological physics of the developing embryo. Cambridge University Press. 2005 [2017-01-27]. ISBN 978-0-521-78337-8. (原始内容存档于2021-01-11).
- Forgács, G. & Newman, Stuart A. Epithelial morphogenesis: gastrulation and neurulation. Biological physics of the developing embryo. Cambridge University Press. 2005. ISBN 978-0-521-78337-8.
- Hart, Nathan H. & Fluck, Richard A. Epiboly and Gastrulation. Capco, David (编). Cytoskeletal mechanisms during animal development. Academic Press. 1995. ISBN 978-0-12-153131-7.
- Knust, Elizabeth. Gastrulation movements. Birchmeier, Walter; Birchmeier, Carmen (编). Epithelial Morphogenesis in Development and Disease. CRC Press. 1999: 152–153. ISBN 978-90-5702-419-1.
- Kunz, Yvette W. Gastrulation. Developmental biology of Teleost fishes. Springer. 2004. ISBN 978-1-4020-2996-7.
- Nation, James L. (编). Gastrulation. Insect physiology and biochemistry. CRC Press. 2009. ISBN 978-0-8493-1181-9.
- Ross, Lawrence M.; Lamperti, Edward D. (编). Human Ontogeny: Gastrulation, Neurulation, and Somite Formation. Atlas of anatomy: general anatomy and musculoskeletal system. Thieme. 2006. ISBN 978-3-13-142081-7.
- Sanes, Dan H. et al. Early embryology of metazoans. Development of the nervous system 2nd. Academic Press. 2006: 1–2 [2017-01-27]. ISBN 978-0-12-618621-5. (原始内容存档于2019-05-14).
- Stanger, Ben Z. & Melton, Douglas A. Development of Endodermal Derivatives in the Lungs, Liver, Pancreas, and Gut. Epstein, Charles J. et al (编). Inborn errors of development: the molecular basis of clinical disorders of morphogenesis. Oxford University Press. 2004. ISBN 978-0-19-514502-1.