模板:Infobox drug/testcases
Type=MAB
編輯 | |
 上方:β-羥基β-甲基丁酸 下方:β-羥基β-甲基丁酸根 | |
| 單克隆抗體 | |
|---|---|
| 種類 | 完整抗體 |
| 臨床資料 | |
| 其他名稱 | Conjugate acid form: β-hydroxyisovaleric acid 3-hydroxyisovaleric acid Conjugate base form: hydroxymethylbutyrate |
| 給藥途徑 | By mouth[1] or nasogastric[2] |
| ATC碼 |
|
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 代謝產物 | HMB-CoA, HMG-CoA, mevalonate, cholesterol, acetyl-CoA, acetoacetate, β-hydroxybutyrate |
| 藥效起始時間 | HMB-FA: 30–60 minutes[1] HMB-Ca: 1–2 hours[1] |
| 生物半衰期 | HMB-FA: 3 hours[1] HMB-Ca: 2.5 hours[1] |
| 排泄途徑 | Renal (10–40% excreted)[1][3] |
| 識別資訊 | |
| CAS號 | 625-08-1 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| 化學資訊 | |
| 化學式 | C5H10O3 |
| 摩爾質量 | 118.13 g·mol−1 |
| 3D模型(JSmol) | |
| 密度 | ~1.1 g/cm3 at 20 °C[4] |
| 熔點 | −80 °C(−112 °F) (glass)[5] |
| 沸點 | 128 °C(262 °F) at 7 mmHg[4][6] |
| |
| |
Type=Vaccine
編輯 | |
 上方:β-羥基β-甲基丁酸 下方:β-羥基β-甲基丁酸根 | |
| 疫苗說明 | |
|---|---|
| 種類 | 不活化 |
| 臨床資料 | |
| 其他名稱 | Conjugate acid form: β-hydroxyisovaleric acid 3-hydroxyisovaleric acid Conjugate base form: hydroxymethylbutyrate |
| 給藥途徑 | By mouth[1] or nasogastric[2] |
| ATC碼 |
|
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 代謝產物 | HMB-CoA, HMG-CoA, mevalonate, cholesterol, acetyl-CoA, acetoacetate, β-hydroxybutyrate |
| 藥效起始時間 | HMB-FA: 30–60 minutes[1] HMB-Ca: 1–2 hours[1] |
| 生物半衰期 | HMB-FA: 3 hours[1] HMB-Ca: 2.5 hours[1] |
| 排泄途徑 | Renal (10–40% excreted)[1][3] |
| 識別資訊 | |
| CAS號 | 625-08-1 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| 化學資訊 | |
| 化學式 | C5H10O3 |
| 摩爾質量 | 118.13 g·mol−1 |
| 3D模型(JSmol) | |
| 密度 | ~1.1 g/cm3 at 20 °C[4] |
| 熔點 | −80 °C(−112 °F) (glass)[5] |
| 沸點 | 128 °C(262 °F) at 7 mmHg[4][6] |
| |
| |
Type=Combo (en:Adderall drugbox)
編輯 | |
 | |
| 組成 | |
|---|---|
| amphetamine aspartate monohydrate | 25% – stimulant (12.5% levo; 12.5% dextro) |
| amphetamine sulfate | 25% – stimulant (12.5% levo; 12.5% dextro) |
| dextroamphetamine saccharate | 25% – stimulant (0% levo; 25% dextro) |
| dextroamphetamine sulfate | 25% – stimulant (0% levo; 25% dextro) |
| 臨床資料 | |
| 商品名 | Adderall, Adderall XR, Mydayis |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601234 |
| 核准狀況 | |
| 依賴性 | Physical: none[7] Psychological: moderate[8] |
| 成癮性 | Moderate |
| 給藥途徑 | Oral, insufflation, rectal, sublingual |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 識別資訊 | |
| CAS號 | 300-62-9(51-64-9) |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| 化學資訊 | |
| 摩爾質量 | 135.20622 g/mol[9] |
| 手性 | Racemic mixture[10] |
| 密度 | .936 g/cm3 at 25 °C[11] |
| 熔點 | 11.3 °C(52.3 °F) (predicted)[12] |
| 沸點 | 203 °C(397 °F) at 760 mmHg[13] |
Amphetamine - English WP drugbox
編輯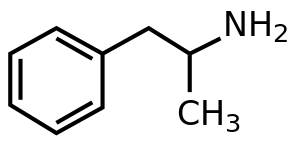 | |
 | |
| 臨床資料 | |
|---|---|
| 讀音 | |
| 商品名 | Adderall, Adzenys XR-ODT, Dyanavel XR, Evekeo, others |
| 其他名稱 | α-methylphenethylamine |
| AHFS/Drugs.com | amphetamine |
| 依賴性 | Physical: none[14] Psychological: moderate[8] |
| 成癮性 | Moderate |
| 給藥途徑 | Medical: oral, intravenous[15] Recreational: oral, insufflation, rectal, intravenous, intramuscular |
| 藥物類別 | CNS stimulant |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 生物利用度 | Oral: 75–100%[16] |
| 血漿蛋白結合率 | 15–40%[17] |
| 藥物代謝 | CYP2D6,[18] DBH,[26][27] FMO3[26][28][29] |
| 代謝產物 | 4-hydroxyamphetamine, 4-hydroxynorephedrine, 4-hydroxyphenylacetone, benzoic acid, hippuric acid, norephedrine, phenylacetone[18][19] |
| 藥效起始時間 | IR dosing: 30–60 minutes[20] XR dosing: 1.5–2 hours[21][22] |
| 生物半衰期 | D-amph: 9–11 hours[18][23] L-amph: 11–14 hours[18][23] pH-dependent: 7–34 hours[24] |
| 作用時間 | IR dosing: 3–6 hours[8][21][25] XR dosing: 8–12 hours[8][21][25] |
| 排泄途徑 | Primarily renal; pH-dependent range: 1–75%[18] |
| 識別資訊 | |
| |
| CAS號 | 300-62-9 |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| 化學資訊 | |
| 化學式 | C9H13N |
| 摩爾質量 | 135.20622 g/mol[9] |
| 3D模型(JSmol) | |
| 手性 | Racemic mixture[10] |
| 密度 | .936 g/cm3 at 25 °C[11] |
| 熔點 | 11.3 °C(52.3 °F) (predicted)[12] |
| 沸點 | 203 °C(397 °F) at 760 mmHg[13] |
| |
| |
Reflist
編輯延伸內容
|
|---|
|