比卡魯胺
比卡魯胺(英語:Bicalutamide)以Casodex(可蘇多錠)等品牌於市面銷售,是種抗雄激素藥物,主要用於治療攝護腺癌。[10]它通常與促性腺激素釋放激素調節劑 (GnRH) 類似物或睪丸切除術(去勢)同時使用,以治療轉移性攝護腺癌 (mPC)。[11][10][12]在較不嚴重的案例,可用它作單一療法,以高劑量治療局部晚期攝護腺癌(LAPC),而無需進行去勢。[4][2][13]比卡魯胺曾被用作治療局限性攝護腺癌 (LPC) 的單一療法,但因臨床試驗結果不佳,核准遭到撤銷。比卡魯胺除用於治療攝護腺癌之外,也被有限地用於治療女性先天性遺傳多毛症和雄激素性脫髮,[14][15]並用於處理男性陰莖異常勃起。[16]此藥物係透過口服方式給藥。[10]
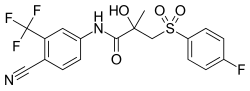 | |
 | |
| 臨床資料 | |
|---|---|
| 讀音 | Bicalutamide: • /ˌbaɪkəˈluːtəmaɪd/[1] • BY-kə-LOO-tə-myde[1] |
| 商品名 | Casodex、Calutex及其他 |
| 其他名稱 | ICI-176,334,ZD-176,334 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697047 |
| 核准狀況 | |
| 懷孕分級 |
|
| 給藥途徑 | 口服給藥[2] |
| 藥物類別 | 非類固醇抗雄激素 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學資料 | |
| 生物利用度 | 容易吸收,絕對生物利用度尚未知[3] |
| 血漿蛋白結合率 | 外消旋體: 96.1%[2] 對映異構: 99.6%[2] (主要與人類血清白蛋白結合)[2] |
| 藥物代謝 | 肝臟 (廣泛性):[4][9] • 羥基化 (CYP3A4) • 葡醣苷酸化 (UGT1A9酶) |
| 代謝產物 | • 比卡魯胺葡醣苷酸 •羥基比卡魯胺 • 羥基比卡魯胺 葡醣苷酸 (全部非活性)[4][2][5][6] |
| 生物半衰期 | 單劑量: 5.8天[7] 持續使用: 7–10天[8] |
| 排泄途徑 | 糞便: 43%[4] 尿液: 34%[4] |
| 識別資訊 | |
| |
| CAS編號 | 90357-06-5( 113299-40-4 ((R)-isomer) 113299-38-0 ((S)-isomer)) |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB配位基ID | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.126.100 |
| 化學資訊 | |
| 化學式 | C18H14F4N2O4S |
| 莫耳質量 | 430.37 g·mol−1 |
| 3D模型(JSmol) | |
| 手性 | 外消旋體 (of (R)- and (S)-對映異構) |
| 熔點 | 191至193 °C(376至379 °F) (實驗性) |
| 沸點 | 650 °C(1,202 °F) (推測) |
| 水溶性 | 0.005 |
| |
| |
使用後對男性的常見副作用有男性乳腺發育、乳房疼痛和潮熱。[10]對男性的其他副作用有女性化和性功能障礙。[17][18]如進行過去勢手術,有些副作用(例如乳腺發育和女性化)會大幅減少。[19]雖然該藥物對女性產生的副作用似乎很少,但目前美國食品藥物管理局 (FDA) 尚未明確批准提供女性使用。[20][10]個體於懷孕期間使用此藥物可能會傷害胎兒。[10]在罹患早期攝護腺癌的男性中,使用比卡魯胺單一療法會增加攝護腺癌以外原因導致死亡的可能性。[21][13]比卡魯胺會導致約1%的使用者出現異常肝臟變化(轉胺酶升高)而須停止用藥。[22][13]罕見的情況有與嚴重肝損傷、[10]嚴重肺毒性、[3]和對光敏感的案例有關聯。[23][24]雖然肝臟發生不良變化的風險很小,仍建議在治療期間進行肝功能檢測。[10]
比卡魯胺是種非類固醇抗雄激素 (NSAA) 類藥物。[3]它透過選擇性阻斷雄激素受體 (AR) 而發揮作用。[25]人體對比卡魯胺的吸收度良好,飲食期間服用並不受影響。[2]藥物的生物半衰期約為一週。[2][10]它在動物體內是種週邊選擇性藥物,但對人類而言,則會穿過血腦屏障並對身體和大腦產生影響。[2][26]
比卡魯胺於1982年取得專利,於1995年獲准用於醫療用途,[27]並已被列入世界衛生組織基本藥物標準清單中。[28]市面已有通用名藥物販售。[29]該藥物在80多個國家(包括大多數已開發國家)流通。[30][31][32]
醫療用途
編輯比卡魯胺主要用於以下適應症:[33]
- 男性轉移性攝護腺癌 (mPC) ,同時攝取促性腺激素釋放激素調節劑 (GnRH) 類似物或是與去勢手術聯合使用,每日攝取50毫克。[22][4][34]
- 男性局部晚期攝護腺癌 (LAPC) 單一療法,每日攝取150毫克(美國並未批准此種用途)[4][2][13][35]
日本核准的治療攝護腺癌,藥物使用劑量為每日80毫克,既可與去勢手術結合使用,也可作單一療法使用。[36][37]
比卡魯胺的仿單標示外使用適應症有:
- 減少男性接受GnRH激動劑治療開始時睪酮激增的影響。[38][39]
- 雄激素依賴性皮膚和毛髮疾病,如女性痤瘡、皮脂分泌旺盛、先天性遺傳多毛症和雄激素性脫髮,以及女性多囊卵巢症候群 (PCOS) 導致的高睪酮水平,通常會與避孕藥聯合使用,比卡魯胺的用量為每日25至50毫克。[14][40][41][42][43][44][45]
- 為跨性別女性進行女性化激素療法,通常與雌激素合併使用,劑量為每日50毫克。[46][47][48][49][50][51][52]
- 男孩週邊性早熟(特別是家族性男性限定性性早熟(睪丸毒性),每日服用12.5至100毫克,與芳香化酶抑制劑(如阿那曲唑)聯合使用。[22][53][54][55][56][57][58]
- 男性陰莖異常勃起,每週攝取50毫克至每隔一天攝取50毫克。[59][60][61][62][3][7][16]
此藥物已被建議用於以下適應症,但效果尚不確定:
配方形式
編輯比卡魯胺在全球80多個國家(大多數為已開發國家)經核准用於治療攝護腺癌。[69][30][70][31][32]有50毫克、80毫克(在日本)[36]和150毫克口服片劑形式。該藥物在至少55個國家註冊為每日150毫克的單一療法,以治療男性局部晚期攝護腺癌,[2]但在美國是一明顯例外,該藥物僅註冊為每日50毫克的劑量,結合去勢使用。[71]
禁忌症
編輯比卡魯胺在美國屬於妊娠X類,即"妊娠禁忌",[22]在"澳大利亞"屬於妊娠D類,即第二大限制級別。[72]因此懷孕期間的女性禁用比卡魯胺,強烈建議性活躍且能夠或可能懷孕的女性僅在採取適當避孕措施的情況下才能服用比卡魯胺。[73][74]目前尚不清楚比卡魯胺是否會進入母乳中,但同樣不建議在個體進行母乳哺育時使用比卡魯胺。[3][22]
對患有嚴重肝病的個體,有證據顯示人體消除比卡魯胺的速度會減慢,由於體內比卡魯胺的水平可能會因此增加,患者需要謹慎。[2][75]比卡魯胺的生物半衰期在腎功能損害患者體中並無變化。[71]
副作用
編輯比卡魯胺的副作用很大程度上取決於性別。在男性中,由於雄激素缺乏症,有不同嚴重程度的副作用會出現,最常見的是乳房疼痛/壓痛和乳腺發育。 在接受比卡魯胺單一藥物治療的男性中,高達80%的男性會出現乳腺發育,超過90%的乳腺發育的嚴重程度為輕度至中度。[76][77]其他與雄性素缺乏類似的副作用有潮熱、性功能障礙(例如性衝動喪失、勃起功能障礙)、憂鬱、疲勞、虛弱和貧血。[78][79][80]
比卡魯胺與肝功能檢查異常結果(例如肝臟酵素水平升高)有關聯。[79][13]建議在治療期間監測肝功能,特別是在最初的幾個月。[13][78]在患有早期攝護腺癌的男性中,發現使用比卡魯胺單一療法會增加非攝護腺癌原因的死亡率。[21][81][13]這些死亡率增加的原因尚不清楚,可能的因素包括雄激素缺乏或比卡魯胺藥物相關毒性。[82][83]
截至2022年,已有10例與比卡魯胺相關的肝毒性病例報告提出。[84][85][86][87]
由於比卡魯胺是一種抗雄激素,理論上存在導致男性胎兒先天性障礙的風險,例如生殖器不明確。[73][74][88][89]由於這種可能的致畸能力,具有生育能力和性活躍的女性於服用比卡魯胺時,應採取避孕措施。[90]
過量
編輯尚未確定人類單次攝取比卡魯胺會導致過量症狀,或被認為會危及生命。[22][91]在臨床試驗中,高達每日600毫克的劑量仍受到良好耐受。[92]
與其他藥物交互作用
編輯比卡魯胺幾乎完全由肝臟的CYP3A4代謝。[4]因此CYP3A4的抑制劑和誘導劑可能會改變藥物在體內的水平。[7]雖然比卡魯胺是由CYP3A4代謝,但沒證據顯示使用劑量為每日150毫克或更少,而同時使用其他引發或抑制CYP3A4的藥物後會發生臨床上顯著的藥物交互作用。[13]
由於比卡魯胺以相對較高的濃度在人體中循環,且具有高度蛋白質結合力,因此有可能取代血漿中華法林、苯妥英、茶鹼和阿斯匹靈等其他具高度蛋白質結合力的藥物。[76][79]
藥理學
編輯藥效學
編輯藥物動力學
編輯此藥物在人體中的絕對生物利用度尚未被完全了解,[2][3]但已知比卡魯胺具有廣泛且吸收良好的特性。其吸收不受患者進行飲食的影響。[3][93]
比卡魯胺的藥物動力學不受同時進食、年齡或體重、腎功能損害或輕至中度肝功能損害的影響。[2][94]然而服用此藥物的日本人,其體內藥物的穩態水準會高於白人的。[2]
化學性質
編輯類似物
編輯第一代非類固醇抗雄激素藥物包括比卡魯胺、氟他胺和尼魯米特,都是合成的非類固醇苯胺(抗雄激素)衍生物,並且彼此具有類似結構。[95]
合成
編輯已有許多文獻提出比卡魯胺的化學合成法。[96][97][98][99][100]首次發表的比卡魯胺的合成過程如下圖所示。[97]
Bicalutamide synthesis[97]
|
歷史
編輯比卡魯胺以及目前上市的所有非類固醇抗雄激素藥物均源自氟他胺的結構修飾,氟他胺本身最初於1967年由先靈葆雅公司合成,作抑菌劑用途,而後偶然發現其具有抗雄激素活性。[101][102][103]比卡魯胺由在帝國化學工業 (ICI) 工作的Tucker及其同事於20世紀80年代從超過2,000種合成化合物篩選後開發而成。[104][105][106][96]此化合物於1982年首次獲得專利,[107]並於1987年6月首次在科學文獻中提出報告。[108]
比卡魯胺為通用名稱,在各種國際性及國家性藥典/名稱登記中均被使用。[109][72][30][110][69][111]
社會與文化
編輯品牌名稱
編輯有Casodex、Cosudex、Calutide、Calumid和Kalumi等。[30][69][112][113]
通用名藥物
編輯比卡魯胺已不受專利保護,市面有眾多通用名藥物流通,[114]而且價格較為便宜。[115][116]
銷售
編輯比卡魯胺(如Casodex)的全球銷售額於2007年達到13億美元的峰值,[117]在2007年失去專利保護之前,它是種"每年銷售達十億美元"的藥物之一。[118][119][120]比卡魯胺仍然是轉移性攝護腺癌經去勢手術後最常用的處方藥。[118]
於2007年到2009的兩年間,在美國開立的NSAA處方箋中,比卡魯胺約佔87.2%,氟他胺佔10.5%,尼魯米特佔2.3%。[121]
研究
編輯目前有將比卡魯胺與5α-還原酶抑制劑非那雄胺和度他雄胺聯合,以治療攝護腺癌的研究。[122][123][124][125][126][127][128]它也與雷洛昔芬(一種選擇性雌激素受體調節物(SERM))聯合用於治療攝護腺癌。[129][130]
有使用抗雄激素藥物治療男性COVID-19的提議,截至2020年5月,使用高劑量比卡魯胺的療法正處於II期臨床試驗中。[131][132]
參見
編輯參考文獻
編輯- ^ 1.0 1.1 Finkel R, Clark MA, Cubeddu LX. Pharmacology. Lippincott Williams & Wilkins. 2009: 481–. ISBN 978-0-7817-7155-9.
- ^ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 Cockshott ID. Bicalutamide: clinical pharmacokinetics and metabolism. Clinical Pharmacokinetics. 2004, 43 (13): 855–878. PMID 15509184. S2CID 29912565. doi:10.2165/00003088-200443130-00003.
- ^ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Dart RC. Medical Toxicology. Lippincott Williams & Wilkins. 2004: 497, 521. ISBN 978-0-7817-2845-4. (原始內容存檔於11 May 2016).
- ^ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Lemke TL, Williams DA. Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. 2008: 121, 1288, 1290. ISBN 978-0-7817-6879-5. (原始內容存檔於2017-09-08).
- ^ Dole EJ, Holdsworth MT. Nilutamide: an antiandrogen for the treatment of prostate cancer. The Annals of Pharmacotherapy. 1997, 31 (1): 65–75. PMID 8997470. S2CID 20347526. doi:10.1177/106002809703100112.
page 67: Currently, information is not available regarding the activity of the major urinary metabolites of bicalutamide, bicalutamide glucuronide, and hydroxybicalutamide glucuronide.
- ^ Schellhammer PF. An evaluation of bicalutamide in the treatment of prostate cancer. Expert Opinion on Pharmacotherapy. September 2002, 3 (9): 1313–28. PMID 12186624. S2CID 32216411. doi:10.1517/14656566.3.9.1313.
The clearance of bicalutamide occurs pre- dominantly by hepatic metabolism and glucuronidation, with excretion of the resulting inactive metabolites in the urine and faces.
- ^ 7.0 7.1 7.2 Skidmore-Roth L. Mosby's 2014 Nursing Drug Reference – Elsevieron VitalSource. Elsevier Health Sciences. 2013-04-17: 193–194 [2016-09-27]. ISBN 978-0-323-22267-9. (原始內容存檔於2023-01-14).
- ^ Jordan VC, Furr BJ. Hormone Therapy in Breast and Prostate Cancer. Springer Science & Business Media. 2010-02-05: 350–. ISBN 978-1-59259-152-7. (原始內容存檔於2016-05-29).
- ^ Grosse L, Campeau AS, Caron S, Morin FA, Meunier K, Trottier J, Caron P, Verreault M, Barbier O. Enantiomer selective glucuronidation of the non-steroidal pure anti-androgen bicalutamide by human liver and kidney: role of the human UDP-glucuronosyltransferase (UGT)1A9 enzyme. Basic & Clinical Pharmacology & Toxicology. August 2013, 113 (2): 92–102. PMC 3815647 . PMID 23527766. doi:10.1111/bcpt.12071.
- ^ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 Bicalutamide. The American Society of Health-System Pharmacists. [2016-12-08]. (原始內容存檔於2016-12-29).
- ^ Wass JA, Stewart PM. Oxford Textbook of Endocrinology and Diabetes. OUP Oxford. 2011-07-28: 1625–. ISBN 978-0-19-923529-2. (原始內容存檔於2016-05-11).
- ^ Shergill I, Arya M, Grange PR, Mundy AR. Medical Therapy in Urology. Springer Science & Business Media. 2010: 40. ISBN 9781848827042. (原始內容存檔於28 October 2014) (英語).
- ^ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 Wellington K, Keam SJ. Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer (PDF). Drugs. 2006, 66 (6): 837–50 [2016-08-13]. PMID 16706554. S2CID 46966712. doi:10.2165/00003495-200666060-00007. (原始內容 (PDF)存檔於2016-08-28).
- ^ 14.0 14.1 Williams H, Bigby M, Diepgen T, Herxheimer A, Naldi L, Rzany B. Evidence-Based Dermatology. John Wiley & Sons. 2009-01-22: 529–. ISBN 978-1-4443-0017-8. (原始內容存檔於2016-05-02).
- ^ Carvalho RM, Santos LD, Ramos PM, Machado CJ, Acioly P, Frattini SC, Barcaui CB, Donda AL, Melo DF. Bicalutamide and the new perspectives for female pattern hair loss treatment: What dermatologists should know. J Cosmet Dermatol. January 2022, 21 (10): 4171–4175. PMID 35032336. S2CID 253239337. doi:10.1111/jocd.14773.
- ^ 16.0 16.1 Yuan J, Desouza R, Westney OL, Wang R. Insights of priapism mechanism and rationale treatment for recurrent priapism. Asian Journal of Andrology. 2008, 10 (1): 88–101. PMID 18087648. doi:10.1111/j.1745-7262.2008.00314.x .
- ^ Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW. Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life. The Journal of Sexual Medicine. 2010, 7 (9): 2996–3010. PMID 20626600. doi:10.1111/j.1743-6109.2010.01902.x.
- ^ Hammerer P, Manka L. Androgen Deprivation Therapy for Advanced Prostate Cancer. Urologic Oncology. Springer International Publishing. 2019: 255–276. ISBN 978-3-319-42622-8. doi:10.1007/978-3-319-42623-5_77.
Bicalutamide is the most widely used antiandrogen in the treatment of prostate cancer. [...] Common side effects [of bicalutamide] include breast enlargement, breast tenderness, hot flashes, and constipation as well as feminization and changes in mood and liver as well as lung toxicity; monitoring of liver enzymes is recommended during treatment (Schellhammer and Davis 2004).
- ^ Droz J, Audisio RA. Management of Urological Cancers in Older People. Springer Science & Business Media. 2012-10-02: 84–. ISBN 978-0-85729-986-4. (原始內容存檔於2016-05-11).
- ^ Shapiro J. Hair Disorders: Current Concepts in Pathophysiology, Diagnosis and Management, An Issue of Dermatologic Clinics. Elsevier Health Sciences. 2012-11-12: 187–. ISBN 978-1-4557-7169-1.
- ^ 21.0 21.1 Jia AY, Spratt DE. Bicalutamide Monotherapy With Radiation Therapy for Localized Prostate Cancer: A Non-Evidence-Based Alternative. Int J Radiat Oncol Biol Phys. June 2022, 113 (2): 316–319. PMID 35569476. S2CID 248765294. doi:10.1016/j.ijrobp.2022.01.037.
Four other randomized trials using BICmono have also raised concerns about either lack of efficacy or even harm from this treatment approach compared with placebo or no hormone therapy. SPCG-6 randomized 1218 patients to either 150 mg of BICmono daily or placebo. In the subset of patients with LPCa managed with observation, survival was significantly worse with BIC than placebo (hazard ratio [HR], 1.47; 95% confidence interval, 1.06-2.03).10 Two other randomized trials were part of the early prostate cancer program,11 which conducted 3 randomized trials that were pooled together to determine the benefit of BICmono (SPCG-6 was one of the 3 trials). Overall, in the combined 8113 patient pooled cohort, after a median follow-up of 7 years, there was no improvement even in progression-free survival from the use of adjuvant BIC in LPCa, and there was a trend for worse overall survival (HR, 1.16; 95% confidence interval, 0.99-1.37; P = .07). [...] Although not in LPCa, NRG/RTOG 9601 demonstrated findings consistent with the prior trials.12 This trial randomized men to postprostatectomy salvage radiation therapy plus placebo versus 150 mg of BICmono daily for 2 years. After a median follow-up of 13 years, the trial showed that there were significantly more grade 3 to 5 cardiac events in the BICmono arm. In patients with less aggressive disease with lower PSAs (prostate-specific antigens; more analogous to LPCa), other-cause mortality was significantly higher in the BICmono arm. In patients with high PSAs >1.5 ng/mL (which with modern molecular positron emission tomography imaging would be expected to have high rates of regional and distant metastatic disease), a survival benefit from the addition of BIC was observed. This is consistent with results from the early prostate cancer studies that showed that only patients with more advanced disease derived benefit from BICmono.10 Thus, all the randomized evidence from 5 trials (Table 1) demonstrates that, in LPCa, BICmono had no clinically significant oncologic activity over placebo/no treatment, and consistent trends with long-term use resulted in worse survival.
- ^ 22.0 22.1 22.2 22.3 22.4 22.5 Casodex- bicalutamide tablet. DailyMed. 2019-09-01 [2020-05-07]. (原始內容存檔於2020-07-27).
- ^ Lee K, Oda Y, Sakaguchi M, Yamamoto A, Nishigori C. Drug-induced photosensitivity to bicalutamide – case report and review of the literature. Photodermatology, Photoimmunology & Photomedicine. May 2016, 32 (3): 161–4. PMID 26663090. S2CID 2761388. doi:10.1111/phpp.12230.
- ^ Lee K, et al. Drug-induced photosensitivity to bicalutamide – case report and review of the literature. Reactions Weekly. 2016, 1612 (1): 161–4. PMID 26663090. S2CID 261402820. doi:10.1007/s40278-016-19790-1.
- ^ Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (antiandrogens): structure-activity relationships. Current Medicinal Chemistry. February 2000, 7 (2): 211–47. PMID 10637363. doi:10.2174/0929867003375371.
- ^ Furr BJ, Tucker H. The preclinical development of bicalutamide: pharmacodynamics and mechanism of action. Urology. January 1996, 47 (1A Suppl): 13–25; discussion 29–32. PMID 8560673. doi:10.1016/S0090-4295(96)80003-3.
- ^ Fischer J, Ganellin CR. Analogue-based Drug Discovery. John Wiley & Sons. 2006: 515 [2017-08-24]. ISBN 9783527607495. (原始內容存檔於2023-01-12) (英語).
- ^ World Health Organization. World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. hdl:10665/325771 . WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Hamilton R. Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. 2015: 381. ISBN 9781284057560.
- ^ 30.0 30.1 30.2 30.3 Bicalutamide – International Drug Names. Drugs.com. [2016-08-13]. (原始內容存檔於2016-09-18).
- ^ 31.0 31.1 Akaza H. [A new anti-androgen, bicalutamide (Casodex), for the treatment of prostate cancer—basic clinical aspects]. Gan to Kagaku Ryoho. Cancer & Chemotherapy. 1999, 26 (8): 1201–7. PMID 10431591 (日語).
- ^ 32.0 32.1 1999 Annual Report and Form 20-F (PDF). AstraZeneca. [2017-07-01]. (原始內容存檔 (PDF)於2017-09-09).
- ^ Bagatelle C, Bremner WJ. Androgens in Health and Disease. Springer Science & Business Media. 2003-05-27: 25–. ISBN 978-1-59259-388-0.
- ^ Klotz L, Schellhammer P. Combined androgen blockade: the case for bicalutamide. Clinical Prostate Cancer. March 2005, 3 (4): 215–9. PMID 15882477. doi:10.3816/cgc.2005.n.002.
- ^ Schellhammer PF, Sharifi R, Block NL, Soloway MS, Venner PM, Patterson AL, Sarosdy MF, Vogelzang NJ, Schellenger JJ, Kolvenbag GJ. Clinical benefits of bicalutamide compared with flutamide in combined androgen blockade for patients with advanced prostatic carcinoma: final report of a double-blind, randomized, multicenter trial. Casodex Combination Study Group. Urology. September 1997, 50 (3): 330–6. PMID 9301693. doi:10.1016/S0090-4295(97)00279-3.
- ^ 36.0 36.1 Suzuki H, Kamiya N, Imamoto T, Kawamura K, Yano M, Takano M, Utsumi T, Naya Y, Ichikawa T. Current topics and perspectives relating to hormone therapy for prostate cancer. International Journal of Clinical Oncology. October 2008, 13 (5): 401–10. PMID 18946750. S2CID 32859879. doi:10.1007/s10147-008-0830-y.
- ^ Usami M, Akaza H, Arai Y, Hirano Y, Kagawa S, Kanetake H, Naito S, Sumiyoshi Y, Takimoto Y, Terai A, Yoshida H, Ohashi Y. Bicalutamide 80 mg combined with a luteinizing hormone-releasing hormone agonist (LHRH-A) versus LHRH-A monotherapy in advanced prostate cancer: findings from a phase III randomized, double-blind, multicenter trial in Japanese patients. Prostate Cancer Prostatic Dis. 2007, 10 (2): 194–201. PMID 17199134. doi:10.1038/sj.pcan.4500934 .
In most countries, bicalutamide is given at a dose of 50 mg when used in combination with an LHRH-A. However, based on pharmacokinetic and pharmacodynamic data, the approved dose of bicalutamide in Japanese men is 80 mg per day.
- ^ Melmed S. Williams Textbook of Endocrinology. Elsevier Health Sciences. 2016-01-01: 752–. ISBN 978-0-323-29738-7.
GnRH analogues, both agonists and antagonists, severely suppress endogenous gonadotropin and testosterone production [...] Administration of GnRH agonists (e.g., leuprolide, goserelin) produces an initial stimulation of gonadotropin and testosterone secretion (known as a "flare"), which is followed in 1 to 2 weeks by GnRH receptor downregulation and marked suppression of gonadotropins and testosterone to castration levels. [...] To prevent the potential complications associated with the testosterone flare, AR antagonists (e.g., bicalutamide) are usually coadministered with a GnRH agonist for men with metastatic prostate cancer.399
- ^ Sugiono M, Winkler MH, Okeke AA, Benney M, Gillatt DA. Bicalutamide vs cyproterone acetate in preventing flare with LHRH analogue therapy for prostate cancer—a pilot study. Prostate Cancer and Prostatic Diseases. 2005, 8 (1): 91–4. PMID 15711607. doi:10.1038/sj.pcan.4500784 .
- ^ Erem C. Update on idiopathic hirsutism: diagnosis and treatment. Acta Clinica Belgica. 2013, 68 (4): 268–74. PMID 24455796. S2CID 39120534. doi:10.2143/ACB.3267.
- ^ Ascenso A, Marques HC. Acne in the adult. Mini Reviews in Medicinal Chemistry. January 2009, 9 (1): 1–10. PMID 19149656. doi:10.2174/138955709787001730.
- ^ Kaur S, Verma P, Sangwan A, Dayal S, Jain VK. Etiopathogenesis and Therapeutic Approach to Adult Onset Acne. Indian Journal of Dermatology. 2016, 61 (4): 403–7. PMC 4966398 . PMID 27512185. doi:10.4103/0019-5154.185703 .
- ^ Lotti F, Maggi M. Hormonal Treatment for Skin Androgen-Related Disorders. European Handbook of Dermatological Treatments. 2015: 1451–1464. ISBN 978-3-662-45138-0. doi:10.1007/978-3-662-45139-7_142.
- ^ Müderris II, Öner G. Hirsutizm Tedavisinde Flutamid ve Bikalutamid Kullanımı [Flutamide and Bicalutamide Treatment in Hirsutism]. Turkiye Klinikleri Journal of Endocrinology-Special Topics. 2009, 2 (2): 110–2 [2019-03-28]. ISSN 1304-0529. (原始內容存檔於2020-07-27) (土耳其語).
- ^ Moretti C, Guccione L, Di Giacinto P, Simonelli I, Exacoustos C, Toscano V, Motta C, De Leo V, Petraglia F, Lenzi A. Combined Oral Contraception and Bicalutamide in Polycystic Ovary Syndrome and Severe Hirsutism: A Double-Blind Randomized Controlled Trial. J. Clin. Endocrinol. Metab. March 2018, 103 (3): 824–838. PMID 29211888. doi:10.1210/jc.2017-01186 .
- ^ Randolph JF. Gender-Affirming Hormone Therapy for Transgender Females. Clin Obstet Gynecol. December 2018, 61 (4): 705–721. PMID 30256230. S2CID 52821192. doi:10.1097/GRF.0000000000000396.
- ^ Fishman SL, Paliou M, Poretsky L, Hembree WC. Endocrine Care of Transgender Adults. Transgender Medicine. Contemporary Endocrinology. 2019: 143–163. ISBN 978-3-030-05682-7. ISSN 2523-3785. S2CID 86772102. doi:10.1007/978-3-030-05683-4_8.
- ^ Neyman A, Fuqua JS, Eugster EA. Bicalutamide as an Androgen Blocker With Secondary Effect of Promoting Feminization in Male-to-Female Transgender Adolescents. The Journal of Adolescent Health. April 2019, 64 (4): 544–546. PMC 6431559 . PMID 30612811. doi:10.1016/j.jadohealth.2018.10.296.
- ^ Gooren LJ. Clinical practice. Care of transsexual persons. The New England Journal of Medicine. March 2011, 364 (13): 1251–1257. PMID 21449788. doi:10.1056/nejmcp1008161.
- ^ Deutsch M. Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People (PDF) 2nd. University of California, San Francisco: Center of Excellence for Transgender Health. 2016-06-17: 28 [2023-06-09]. (原始內容存檔 (PDF)於2023-05-30).
- ^ Vincent B. Transgender Health: A Practitioner's Guide to Binary and Non-Binary Trans Patient Care. Jessica Kingsley Publishers. 2018-06-21: 158– [2019-01-01]. ISBN 978-1-78450-475-5. (原始內容存檔於2023-01-14).
- ^ Wierckx K, Gooren L, T'Sjoen G. Clinical review: Breast development in trans women receiving cross-sex hormones. The Journal of Sexual Medicine. May 2014, 11 (5): 1240–1247. PMID 24618412. doi:10.1111/jsm.12487.
- ^ Schoelwer M, Eugster EA. Treatment of Peripheral Precocious Puberty. Puberty from Bench to Clinic. Endocrine Development 29. 2015: 230–239. ISBN 978-3-318-02788-4. PMC 5345994 . PMID 26680582. doi:10.1159/000438895.
- ^ Haddad NG, Eugster EA. Peripheral precocious puberty including congenital adrenal hyperplasia: causes, consequences, management and outcomes. Best Practice & Research. Clinical Endocrinology & Metabolism. June 2019, 33 (3): 101273. PMID 31027974. S2CID 135410503. doi:10.1016/j.beem.2019.04.007. hdl:1805/19111 .
- ^ Haddad NG, Eugster EA. Peripheral Precocious Puberty: Interventions to Improve Growth. Handbook of Growth and Growth Monitoring in Health and Disease. 2012: 1199–1212. ISBN 978-1-4419-1794-2. doi:10.1007/978-1-4419-1795-9_71.
- ^ Zacharin M. Disorders of Puberty: Pharmacotherapeutic Strategies for Management. Pediatric Pharmacotherapy. Handbook of Experimental Pharmacology 261. 2019: 507–538. ISBN 978-3-030-50493-9. ISSN 0171-2004. PMID 31144045. S2CID 169040406. doi:10.1007/164_2019_208.
- ^ Kliegman RM, Stanton B, St Geme J, Schor NF. Nelson Textbook of Pediatrics. Elsevier Health Sciences. 17 April 2015: 2661– [2016-09-27]. ISBN 978-0-323-26352-8. (原始內容存檔於2023-01-12).
- ^ Jameson JL, De Groot LJ. Edndocrinology: Adult and Pediatric. Elsevier Health Sciences. 25 February 2015: 2425–2426, 2139. ISBN 978-0-323-32195-2.
- ^ Levey HR, Kutlu O, Bivalacqua TJ. Medical management of ischemic stuttering priapism: a contemporary review of the literature. Asian Journal of Andrology. January 2012, 14 (1): 156–163. PMC 3753435 . PMID 22057380. doi:10.1038/aja.2011.114.
- ^ Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R. Priapism: pathogenesis, epidemiology, and management. The Journal of Sexual Medicine. January 2010, 7 (1 Pt 2): 476–500. PMID 20092449. doi:10.1111/j.1743-6109.2009.01625.x.
- ^ Chow K, Payne S. The pharmacological management of intermittent priapismic states. BJU International. December 2008, 102 (11): 1515–1521. PMID 18793304. S2CID 35399393. doi:10.1111/j.1464-410X.2008.07951.x .
- ^ Dahm P, Rao DS, Donatucci CF. Antiandrogens in the treatment of priapism. Urology. January 2002, 59 (1): 138. PMID 11796309. doi:10.1016/S0090-4295(01)01492-3.
- ^ Gooren LJ. Clinical review: Ethical and medical considerations of androgen deprivation treatment of sex offenders. The Journal of Clinical Endocrinology & Metabolism. 2011, 96 (12): 3628–37. PMID 21956411. doi:10.1210/jc.2011-1540 .
- ^ Giltay EJ, Gooren LJ. Potential side effects of androgen deprivation treatment in sex offenders. The Journal of the American Academy of Psychiatry and the Law. 2009, 37 (1): 53–8. PMID 19297634.
- ^ Khan O, Mashru A. The efficacy, safety and ethics of the use of testosterone-suppressing agents in the management of sex offending. Current Opinion in Endocrinology, Diabetes and Obesity. 2016, 23 (3): 271–8. PMID 27032060. S2CID 43286710. doi:10.1097/MED.0000000000000257.
- ^ Dangerous Sex Offenders: A Task Force Report of the American Psychiatric Association. American Psychiatric Pub. 1999: 111–. ISBN 978-0-89042-280-9.
- ^ Houts FW, Taller I, Tucker DE, Berlin FS. Androgen deprivation treatment of sexual behavior. Advances in Psychosomatic Medicine. 2011, 31: 149–63. ISBN 978-3-8055-9825-5. PMID 22005210. doi:10.1159/000330196.
- ^ Rousseau L, Couture M, Dupont A, Labrie F, Couture N. Effect of combined androgen blockade with an LHRH agonist and flutamide in one severe case of male exhibitionism. The Canadian Journal of Psychiatry. 1990, 35 (4): 338–41. PMID 2189544. S2CID 28970865. doi:10.1177/070674379003500412.
- ^ 69.0 69.1 69.2 Swiss Pharmaceutical Society (編). Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000: 123–. ISBN 978-3-88763-075-1. (原始內容存檔於2016-04-24).
- ^ Sweetman SC. Martindale: The Complete Drug Reference. Pharmaceutical Press. 2011: 750–751 [2016-09-27]. ISBN 978-0-85369-933-0. (原始內容存檔於2023-01-14).
- ^ 71.0 71.1 Chabner BA, Longo DL. Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott Williams & Wilkins. 2010-11-08: 679–680 [2016-09-27]. ISBN 978-1-60547-431-1. (原始內容存檔於2023-01-10).
From a structural standpoint, antiandrogens are classified as steroidal, including cyproterone [acetate] (Androcur) and megestrol [acetate], or nonsteroidal, including flutamide (Eulexin, others), bicalutamide (Casodex), and nilutamide (Nilandron). The steroidal antiandrogens are rarely used.
- ^ 72.0 72.1 COSUDEX® (bicalutamide) 150 mg tablets. TGA. (原始內容存檔於2016-09-14).
- ^ 73.0 73.1 Iswaran TJ, Imai M, Betton GR, Siddall RA. An overview of animal toxicology studies with bicalutamide (ICI 176,334). The Journal of Toxicological Sciences. May 1997, 22 (2): 75–88. PMID 9198005. doi:10.2131/jts.22.2_75 .
- ^ 74.0 74.1 Smith RE. Medicinal Chemistry – Fusion of Traditional and Western Medicine. Bentham Science Publishers. 2013-04-04: 306–. ISBN 978-1-60805-149-6. (原始內容存檔於2016-05-29).
- ^ Skeel RT, Khleif SN. Handbook of Cancer Chemotherapy. Lippincott Williams & Wilkins. 2011: 724–. ISBN 9781608317820. (原始內容存檔於2016-05-29).
- ^ 76.0 76.1 Wirth MP, Hakenberg OW, Froehner M. Antiandrogens in the treatment of prostate cancer. European Urology. February 2007, 51 (2): 306–13; discussion 314. PMID 17007995. doi:10.1016/j.eururo.2006.08.043.
- ^ Wellington K, Keam SJ. Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer. Drugs. 2006, 66 (6): 837–50. PMID 16706554. S2CID 46966712. doi:10.2165/00003495-200666060-00007.
- ^ 78.0 78.1 Lehne RA. Pharmacology for Nursing Care. Elsevier Health Sciences. 2013: 1297– [2016-09-27]. ISBN 978-1-4377-3582-6. (原始內容存檔於2023-01-10).
- ^ 79.0 79.1 79.2 Kolvenbag GJ, Blackledge GR. Worldwide activity and safety of bicalutamide: a summary review. Urology. January 1996, 47 (1A Suppl): 70–9; discussion 80–4. PMID 8560681. doi:10.1016/s0090-4295(96)80012-4.
Bicalutamide is a new antiandrogen that offers the convenience of once-daily administration, demonstrated activity in prostate cancer, and an excellent safety profile. Because it is effective and offers better tolerability than flutamide, bicalutamide represents a valid first choice for antiandrogen therapy in combination with castration for the treatment of patients with advanced prostate cancer.
- ^ Resnick MI, Thompson IM. Advanced Therapy of Prostate Disease. PMPH-USA. 2000: 379–. ISBN 978-1-55009-102-1. (原始內容存檔於2016-06-10).
- ^ Iversen P, Johansson JE, Lodding P, Lukkarinen O, Lundmo P, Klarskov P, Tammela TL, Tasdemir I, Morris T, Carroll K. Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3-year median followup from the Scandinavian Prostate Cancer Group Study Number 6. The Journal of Urology. November 2004, 172 (5 Pt 1): 1871–6. PMID 15540741. doi:10.1097/01.ju.0000139719.99825.54.
- ^ Wellington K, Keam SJ. Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer. Drugs. 2006, 66 (6): 837–50. PMID 16706554. S2CID 46966712. doi:10.2165/00003495-200666060-00007.
- ^ Iversen P, Johansson JE, Lodding P, Kylmälä T, Lundmo P, Klarskov P, Tammela TL, Tasdemir I, Morris T, Armstrong J. Bicalutamide 150 mg in addition to standard care for patients with early non-metastatic prostate cancer: updated results from the Scandinavian Prostate Cancer Period Group-6 Study after a median follow-up period of 7.1 years. Scandinavian Journal of Urology and Nephrology. 2006, 40 (6): 441–52. PMID 17130095. S2CID 25862814. doi:10.1080/00365590601017329.
- ^ Trüeb RM, Luu NC, Uribe NC, Régnier A. Comment on: Bicalutamide and the new perspectives for female pattern hair loss treatment: What dermatologists should know. J Cosmet Dermatol. December 2022, 21 (12): 7200–7201. PMID 35332669. S2CID 247677549. doi:10.1111/jocd.14936.
Indeed, due to the minimal biological importance of androgens in women, the adverse effects of bicalutamide are few. And yet, bicalutamide has been associated with elevated liver enzymes, and as of 2021, there have been 10 case reports of liver toxicity associated with bicalutamide, with fatality occurring in 2 cases.2
- ^ Gretarsdottir HM, Bjornsdottir E, Bjornsson ES. Bicalutamide-Associated Acute Liver Injury and Migratory Arthralgia: A Rare but Clinically Important Adverse Effect. Case Reports in Gastroenterology. 2018, 12 (2): 266–70. ISSN 1662-0631. doi:10.1159/000485175 . hdl:20.500.11815/1492 .
- ^ Hussain S, Haidar A, Bloom RE, Zayouna N, Piper MH, Jafri SM. Bicalutamide-induced hepatotoxicity: A rare adverse effect. Am J Case Rep. 2014, 15: 266–70. PMC 4068966 . PMID 24967002. doi:10.12659/AJCR.890679.
- ^ Yun GY, Kim SH, Kim SW, Joo JS, Kim JS, Lee ES, Lee BS, Kang SH, Moon HS, Sung JK, Lee HY, Kim KH. Atypical onset of bicalutamide-induced liver injury. World J. Gastroenterol. April 2016, 22 (15): 4062–5. PMC 4823258 . PMID 27099451. doi:10.3748/wjg.v22.i15.4062 .
- ^ Sex Differences in the Human Brain, their underpinnings and implications. Elsevier. 2010-12-03: 44–45. ISBN 978-0-444-53631-0. (原始內容存檔於2016-05-26).
- ^ Paoletti R. Chemistry and Brain Development: Proceedings of the Advanced Study Institute on "Chemistry of Brain Development," held in Milan, Italy, September 9–19, 1970. Springer Science & Business Media. 6 December 2012: 218– [2016-09-27]. ISBN 978-1-4684-7236-3. (原始內容存檔於2023-01-14).
- ^ Papadimitriou K, Anagnostis P, Goulis DG. The challenging role of antiandrogens in the management of polycystic ovary syndrome. Polycystic Ovary Syndrome. Elsevier. 2022: 297–314. ISBN 9780128230459. S2CID 244697776. doi:10.1016/B978-0-12-823045-9.00013-4.
- ^ Nurse Practitioner's Drug Handbook. Springhouse Corp. 2000 [2016-09-27]. ISBN 9780874349979. (原始內容存檔於2023-01-14).
- ^ Tyrrell CJ, Iversen P, Tammela T, Anderson J, Björk T, Kaisary AV, Morris T. Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration. BJU International. September 2006, 98 (3): 563–72. PMID 16771791. S2CID 41672303. doi:10.1111/j.1464-410X.2006.06275.x.
- ^ Weber GF. Molecular Therapies of Cancer. Springer. 2015-07-22: 318–. ISBN 978-3-319-13278-5.
Compared to flutamide and nilutamide, bicalutamide has a 2-fold increased affinity for the Androgen Receptor, a longer half-life, and substantially reduced toxicities. Based on a more favorable safety profile relative to flutamide, bicalutamide is indicated for use in combination therapy with a Gonadotropin Releasing Hormone analog for the treatment of advanced metastatic prostate carcinoma.
- ^ Denis L, Mahler C. Pharmacodynamics and pharmacokinetics of bicalutamide: defining an active dosing regimen. Urology. January 1996, 47 (1A Suppl): 26–8; discussion 29–32. PMID 8560674. doi:10.1016/S0090-4295(96)80004-5.
- ^ Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, He Y, Hwang DJ, Dalton JT, Miller DD. Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. Journal of Medicinal Chemistry. June 2009, 52 (12): 3597–617. PMID 19432422. doi:10.1021/jm900280m.
[C]linically relevant antiandrogens currently are nonsteroidal anilide derivatives. Antiandrogens used for prostate cancer include the monoarylpropionamide flutamide (1) (a prodrug of hydroxyflutamide (2)),29–31 the hydantoin nilutamide(3),32–34 and the diarylpropionamide bicalutamide (4) (Chart1).35–37
- ^ 96.0 96.1 McPherson EM. Pharmaceutical Manufacturing Encyclopedia 3rd. William Andrew Publishing. 2013-10-22: 627, 1695. ISBN 978-0-8155-1856-3. (原始內容存檔於2016-06-09).
- ^ 97.0 97.1 97.2 Tucker H, Crook JW, Chesterson GJ. Nonsteroidal antiandrogens. Synthesis and structure-activity relationships of 3-substituted derivatives of 2-hydroxypropionanilides. Journal of Medicinal Chemistry. 1988, 31 (5): 954–9. PMID 3361581. doi:10.1021/jm00400a011.
- ^ James KD, Ekwuribe NN. A Two-step Synthesis of the Anti-cancer Drug (R,S)-Bicalutamide. Synthesis. 2002, 2002 (7): 850–2. doi:10.1055/s-2002-28508.
- ^ US application 2006/0041161,Pizzetti E, Vigano E, Lussana M, Landonio E,「Procedure for the synthesis of bicalutamide」,發表於2006-02-23 網際網路檔案館的存檔,存檔日期2018-01-04.
- ^ Chand M, Shukla AK. Novel Synthesis of Bicalutamide Drug Substance and their Impurities using Imidazolium Type of Ionic Liquid (報告). 2012. SSRN 2160199 . doi:10.2139/ssrn.2160199 .
- ^ Diamanti-Kandarakis E. Current aspects of antiandrogen therapy in women. Current Pharmaceutical Design. September 1999, 5 (9): 707–23 [2016-09-27]. PMID 10495361. doi:10.2174/1381612805666230111201150. (原始內容存檔於2023-01-10).
Several trials demonstrated complete clearing of acne with flutamide [62,77]. Flutamide used in combination with an [oral contraceptive], at a dose of 500mg/d, flutamide caused a dramatic decrease (80%) in total acne, seborrhea and hair loss score after only 3 months of therapy [53]. When used as a monotherapy in lean and obese PCOS, it significantly improves the signs of hyperandrogenism, hirsutism and particularly acne [48]. [...] flutamide 500mg/d combined with an [oral contraceptive] caused an increase in cosmetically acceptable hair density, in sex of seven women suffering from diffuse androgenetic alopecia [53].
- ^ Denis LJ, Griffiths K, Kaisary AV, Murphy GP. Textbook of Prostate Cancer: Pathology, Diagnosis and Treatment: Pathology, Diagnosis and Treatment. CRC Press. 1999-03-01: 55,279–280. ISBN 978-1-85317-422-3. (原始內容存檔於2016-06-03).
- ^ Elks J. The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 2014-11-14: 573–. ISBN 978-1-4757-2085-3. (原始內容存檔於2016-05-15).
- ^ Furr BJ. Research on reproductive medicine in the pharmaceutical industry. Hum Fertil (Camb). 1998, 1 (1): 56–63. PMID 11844311. doi:10.1080/1464727982000198131.
- ^ Furr BJ. Casodex: preclinical studies and controversies. Annals of the New York Academy of Sciences. June 1995, 761 (1): 79–96. Bibcode:1995NYASA.761...79F. PMID 7625752. S2CID 37242269. doi:10.1111/j.1749-6632.1995.tb31371.x.
- ^ Cadilla R, Turnbull P. Selective androgen receptor modulators in drug discovery: medicinal chemistry and therapeutic potential. Curr Top Med Chem. 2006, 6 (3): 245–70 [2019-12-11]. PMID 16515480. doi:10.2174/156802606776173456. (原始內容存檔於2020-08-18).
- ^ Engel J, Kleemann A, Kutscher B, Reichert D. Pharmaceutical Substances: Syntheses, Patents and Applications of the most relevant APIs 5th. Thieme. 2009: 153–154. ISBN 978-3-13-179275-4.
- ^ Furr BJ, Valcaccia B, Curry B, Woodburn JR, Chesterson G, Tucker H. ICI 176,334: a novel non-steroidal, peripherally selective antiandrogen. The Journal of Endocrinology. June 1987, 113 (3): R7–9. PMID 3625091. doi:10.1677/joe.0.113r007.
- ^ Bicalutamide. Kyoto Encyclopedia of Genes and Genomes (KEGG). (原始內容存檔於26 November 2016).
- ^ Morton IK, Hal JM. Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 2012-12-06: 51–. ISBN 978-94-011-4439-1. (原始內容存檔於2016-05-14).
- ^ Ganellin CR, Triggle DJ. Dictionary of Pharmacological Agents. CRC Press. 1996-11-21: 570–. ISBN 978-0-412-46630-4. (原始內容存檔於2016-05-07).
- ^ Ferraro MB, Orendt AM, Facelli JC. Parallel Genetic Algorithms for Crystal Structure Prediction: Successes and Failures in Predicting Bicalutamide Polymorphs. Huang D, Jo K, Lee H, Kang H, Bevilacqua V (編). Emerging Intelligent Computing Technology and Applications: 5th International Conference on Intelligent Computing, ICIC 2009 Ulsan, South Korea, September 16–19, 2009 Proceedings. Lecture Notes in Computer Science 5754. Springer. 2009-09-19: 120– [2016-09-27]. ISBN 978-3-642-04070-2. doi:10.1007/978-3-642-04070-2_14. (原始內容存檔於2023-01-14).
- ^ Dhas NL, Ige PP, Kudarha RR. Design, optimization and in-vitro study of folic acid conjugated-chitosan functionalized PLGA nanoparticle for delivery of bicalutamide in prostate cancer. Powder Technology. 2015, 283: 234–245. doi:10.1016/j.powtec.2015.04.053.
- ^ Moul JW. Twenty years of controversy surrounding combined androgen blockade for advanced prostate cancer. Cancer. August 2009, 115 (15): 3376–8. PMID 19484788. S2CID 5670663. doi:10.1002/cncr.24393 .
- ^ Stuhan MA. Understanding Pharmacology for Pharmacy Technicians. ASHP. 2 April 2013: 268– [2016-11-05]. ISBN 978-1-58528-360-6. (原始內容存檔於2023-01-14).
- ^ Allan GF, Sui Z. Therapeutic androgen receptor ligands. Nucl Recept Signal. 2003, 1: e009. PMC 1402218 . PMID 16604181. doi:10.1621/nrs.01009.
- ^ Annual Report and Form 20-F 2007 (PDF). AstraZeneca. [2024-05-09]. (原始內容存檔 (PDF)於2021-03-06).
- ^ 118.0 118.1 Campbell T. Slowing Sales for Johnson & Johnson's Zytiga May Be Good News for Medivation. The Motley Fool. 2014-01-22 [2016-07-20]. (原始內容存檔於2016-08-26).
[...] the most commonly prescribed treatment for metastatic castration resistant prostate cancer: bicalutamide. That was sold as AstraZeneca's billion-dollar-a-year drug Casodex before losing patent protection in 2008. AstraZeneca still generates a few hundred million dollars in sales from Casodex, [...]
- ^ Actavis Generic Prostate Cancer Drug Bicalutamide First to Market in UK, Germany, France. Press Release. AstraZeneca, Actavis. 2008-07-10 [2024-05-09]. (原始內容存檔於2021-08-28).
- ^ Hermkens PH, Kamp S, Lusher S, Veeneman GH. Non-steroidal steroid receptor modulators. IDrugs. July 2006, 9 (7): 488–94. PMID 16821162. doi:10.2174/0929867053764671.
- ^ Chang S, Bicalutamide BPCA Drug Use Review in the Pediatric Population (PDF), U.S. Department of Health and Human Service, 2010-03-10 [2016-07-20], (原始內容存檔 (PDF)於2016-10-24)
- ^ Wang LG, Mencher SK, McCarron JP, Ferrari AC. The biological basis for the use of an anti-androgen and a 5-alpha-reductase inhibitor in the treatment of recurrent prostate cancer: Case report and review. Oncology Reports. 2004, 11 (6): 1325–9. PMID 15138573. doi:10.3892/or.11.6.1325.
- ^ Tay MH, Kaufman DS, Regan MM, Leibowitz SB, George DJ, Febbo PG, Manola J, Smith MR, Kaplan ID, Kantoff PW, Oh WK. Finasteride and bicalutamide as primary hormonal therapy in patients with advanced adenocarcinoma of the prostate. Annals of Oncology. 2004, 15 (6): 974–8. PMID 15151957. doi:10.1093/annonc/mdh221 .
- ^ Merrick GS, Butler WM, Wallner KE, Galbreath RW, Allen ZA, Kurko B. Efficacy of neoadjuvant bicalutamide and dutasteride as a cytoreductive regimen before prostate brachytherapy. Urology. 2006, 68 (1): 116–20. PMID 16844453. doi:10.1016/j.urology.2006.01.061.
- ^ Sartor O, Gomella LG, Gagnier P, Melich K, Dann R. Dutasteride and bicalutamide in patients with hormone-refractory prostate cancer: the Therapy Assessed by Rising PSA (TARP) study rationale and design. The Canadian Journal of Urology. 2009, 16 (5): 4806–12. PMID 19796455.
- ^ Chu FM, Sartor O, Gomella L, Rudo T, Somerville MC, Hereghty B, Manyak MJ. A randomised, double-blind study comparing the addition of bicalutamide with or without dutasteride to GnRH analogue therapy in men with non-metastatic castrate-resistant prostate cancer. European Journal of Cancer. 2015, 51 (12): 1555–69. PMID 26048455. doi:10.1016/j.ejca.2015.04.028.
- ^ Gaudet M, Vigneault É, Foster W, Meyer F, Martin AG. Randomized non-inferiority trial of Bicalutamide and Dutasteride versus LHRH agonists for prostate volume reduction prior to I-125 permanent implant brachytherapy for prostate cancer. Radiotherapy and Oncology. 2016, 118 (1): 141–7. PMID 26702991. doi:10.1016/j.radonc.2015.11.022.
- ^ Dijkstra S, Witjes WP, Roos EP, Vijverberg PL, Geboers AD, Bruins JL, Smits GA, Vergunst H, Mulders PF. The AVOCAT study: Bicalutamide monotherapy versus combined bicalutamide plus dutasteride therapy for patients with locally advanced or metastatic carcinoma of the prostate-a long-term follow-up comparison and quality of life analysis. SpringerPlus. 2016, 5: 653. PMC 4870485 . PMID 27330919. doi:10.1186/s40064-016-2280-8 .
- ^ Fujimura T, Takayama K, Takahashi S, Inoue S. Estrogen and Androgen Blockade for Advanced Prostate Cancer in the Era of Precision Medicine. Cancers. January 2018, 10 (2): 29. PMC 5836061 . PMID 29360794. doi:10.3390/cancers10020029 .
- ^ Ho TH, Nunez-Nateras R, Hou YX, Bryce AH, Northfelt DW, Dueck AC, et al. A Study of Combination Bicalutamide and Raloxifene for Patients With Castration-Resistant Prostate Cancer. Clinical Genitourinary Cancer. April 2017, 15 (2): 196–202.e1. PMID 27771244. S2CID 19043552. doi:10.1016/j.clgc.2016.08.026.
- ^ McCoy J, Wambier CG, Vano-Galvan S, Shapiro J, Sinclair R, Müller Ramos P, Washenik K, Andrade M, Herrera S, Goren A. Racial Variations in COVID-19 Deaths May Be Due to Androgen Receptor Genetic Variants Associated with Prostate Cancer and Androgenetic Alopecia. Are Anti-Androgens a Potential Treatment for COVID-19?. J Cosmet Dermatol. April 2020, 19 (7): 1542–1543. PMC 7267367 . PMID 32333494. doi:10.1111/jocd.13455 .
- ^ A Phase II Trial to Promote Recovery from COVID-19 with Endocrine Therapy. 2021-03-02 [2024-05-09]. (原始內容存檔於2020-07-26).
延伸閱讀
編輯- Bicalutamide. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2017 [2024-05-09]. PMID 31643303. NBK547970. (原始內容存檔於2016-11-23) –透過NCBI Bookshelf.
- Blackledge GR. Clinical progress with a new antiandrogen, Casodex (bicalutamide). European Urology. 1996, 29 (Suppl 2): 96–104. PMID 8717470. doi:10.1159/000473847.
- Cockshott ID. Bicalutamide: clinical pharmacokinetics and metabolism. Clinical Pharmacokinetics. 2004, 43 (13): 855–78. PMID 15509184. S2CID 29912565. doi:10.2165/00003088-200443130-00003.
- Fradet Y. Bicalutamide (Casodex) in the treatment of prostate cancer. Expert Review of Anticancer Therapy. February 2004, 4 (1): 37–48. PMID 14748655. S2CID 34153031. doi:10.1586/14737140.4.1.37.
- Furr BJ. Casodex: preclinical studies and controversies. Annals of the New York Academy of Sciences. June 1995, 761 (1): 79–96. Bibcode:1995NYASA.761...79F. PMID 7625752. S2CID 37242269. doi:10.1111/j.1749-6632.1995.tb31371.x.
- Furr BJ, Tucker H. The preclinical development of bicalutamide: pharmacodynamics and mechanism of action. Urology. January 1996, 47 (1A Suppl): 13–25; discussion 29–32. PMID 8560673. doi:10.1016/S0090-4295(96)80003-3.
- Kolvenbag GJ, Blackledge GR. Worldwide activity and safety of bicalutamide: a summary review. Urology. January 1996, 47 (1A Suppl): 70–9; discussion 80–4. PMID 8560681. doi:10.1016/s0090-4295(96)80012-4.
- Schellhammer PF, Davis JW. An evaluation of bicalutamide in the treatment of prostate cancer. Clinical Prostate Cancer. March 2004, 2 (4): 213–9. PMID 15072604. doi:10.3816/CGC.2004.n.002.
- Tucker H, Crook JW, Chesterson GJ. Nonsteroidal antiandrogens. Synthesis and structure-activity relationships of 3-substituted derivatives of 2-hydroxypropionanilides. J. Med. Chem. 1988, 31 (5): 954–9. PMID 3361581. doi:10.1021/jm00400a011.
- Wellington K, Keam SJ. Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer. Drugs. 2006, 66 (6): 837–50. PMID 16706554. S2CID 46966712. doi:10.2165/00003495-200666060-00007.